
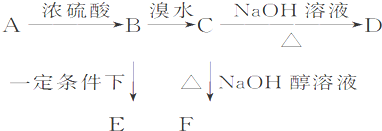
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��| �Ҵ� |
| �� |
| �Ҵ� |
| �� |


 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| NaOH |
| NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�











�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���������뽭��ѧ��һ��ѧ����ĩģ�⿼�Ի�ѧ�Ծ����������� ���ͣ������
��֪�л���A��B��C��D��E��F������ת����ϵ��A�IJ����Ǻ���һ������ʯ�ͻ�������ˮƽ�ı�־��D��ʹʯ����Һ��죻E�Dz�����ˮ�Ҿ�����ζ����ɫҺ�壬��Է���������C��2����F�Ǹ߷��Ӿۺ����������ʳƷ��װ���������ͼ��ϵ�ش����⣺
�Ű�Ҫ��ش��������⣺
��д��A��C�Ľṹ��ʽ��A ��C ��
��д��B��D�й����ŵ����ƣ�B ��D ��
��д����Ӧ�ڵķ�Ӧ����ʽ��
�����й���C��һ��ͬϵ���ȩ��˵������ȷ����__________������ĸ����
a����ȩ�Ľṹ��ʽΪHCHO��������Ϊ��CHO
b����ȩ��ȫȼ�պ�IJ���ΪCO2��H2O
c����ȩ��������������ͬ�Ĺ����ţ�����Һ���������Ʊ���Cu(OH)2����Һ��Ӧ
d����ȩ��ˮ��Һ����ɱ�����������ã����Դ�������ʳƷ�ӹ�ҵ
��A�뱽����ʯ�ͻ�������Ҫ��Ʒ����һ��������A����ת�����ɱ�����Ҫ��ش��������⣺
�ٱ����Է���ȡ����Ӧ��д���ɱ��Ʊ��屽�Ļ�ѧ��Ӧ����ʽ��
�ڴ������屽����ɫ��״Һ�壬ʵ�����ƵõĴ��屽ͨ�����ܽ���Br2�ʺ�ɫ�����Լ����Լ� ��ȥ����Ӧ����ʽΪ ���ó��Ӳ���������IJ��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������и�һ��ѧ����ĩģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
��֪�л���A��B��C��D��E��F������ת����ϵ��A�IJ����Ǻ���һ������ʯ�ͻ�������ˮƽ�ı�־��D��ʹʯ����Һ��죻E�Dz�����ˮ�Ҿ�����ζ����ɫҺ�壬��Է���������C��2����F�Ǹ߷��Ӿۺ����������ʳƷ��װ���������ͼ��ϵ�ش����⣺

�Ű�Ҫ��ش��������⣺
��д��A��C�Ľṹ��ʽ��A ��C ��
��д��B��D�й����ŵ����ƣ�B ��D ��
��д����Ӧ�ڵķ�Ӧ����ʽ��
�����й���C��һ��ͬϵ���ȩ��˵������ȷ����__________������ĸ����
a����ȩ�Ľṹ��ʽΪHCHO��������Ϊ��CHO
b����ȩ��ȫȼ�պ�IJ���ΪCO2��H2O
c����ȩ��������������ͬ�Ĺ����ţ�����Һ���������Ʊ���Cu(OH)2����Һ��Ӧ
d����ȩ��ˮ��Һ����ɱ�����������ã����Դ�������ʳƷ�ӹ�ҵ
��A�뱽����ʯ�ͻ�������Ҫ��Ʒ����һ��������A����ת�����ɱ�����Ҫ��ش��������⣺
�ٱ����Է���ȡ����Ӧ��д���ɱ��Ʊ��屽�Ļ�ѧ��Ӧ����ʽ��
�ڴ������屽����ɫ��״Һ�壬ʵ�����ƵõĴ��屽ͨ�����ܽ���Br2�ʺ�ɫ�����Լ����Լ� ��ȥ����Ӧ����ʽΪ ���ó��Ӳ���������IJ��������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com