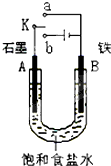
ij����С��ͬѧ����ͼIװ�ý���ʵ�飬�Իش��������⣺
(1)����ʼʱ����K��a���ӣ���B���ĵ缫��ӦʽΪ___________________
(2)����ʼʱ����K��b���ӣ����ܷ�Ӧ�����ӷ���ʽΪ_____________________�й�����ʵ�飬����˵����ȷ����_________������ţ���
����Һ��Na
+��A���ƶ�
�ڴ�A���ݳ���������ʹʪ���KI-������ֽ����
�۷�Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ��
������״����B������2.24 L���壬����Һ��ת��0.2 mol����
(3)��С��ͬѧģ�¹�ҵ�������ӽ���Ĥ�����ռ��ԭ������������ͼ��װ�õ���������Һ����ȡ������������������������ء�
�ٸõ��۹���ʱ��ͨ�������ӽ���Ĥ��������_______������ڡ�����С�ڡ����ڡ���ͨ�������ӽ���Ĥ����������
��ͨ�翪ʼ������������ҺpH�����������ԭ��___________________________��
(4)�ྦྷ����Ҫ����SiHCl
3��ԭ�����������丱����SiCl
4���ۺ������ܵ��㷺��ע��
��SiCl
4���������̿�ڣ�����ά��Ҫԭ����ͬ��������Ϊ������SiCl
4��H
2��O
2��Ӧ�����������֣���ѧ����ʽΪ________________________��
��SiCl
4��ת��ΪSiHCl
3��ѭ��ʹ�á�һ�������£���20 L�����ܱ������еķ�Ӧ��
3SiCl
4(g)+2H
2(g)+Si(s)

4SiHCl
3(g)���ﵽƽ���H
2��SiHCl
3�����ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020 mol/L����H
2ȫ����Դ��ͼ�����ӽ���Ĥ���ĵ�������������������������Ϊ______kg��


 4SiHCl3(g)���ﵽƽ���H2��SiHCl3�����ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020 mol/L����H2ȫ����Դ��ͼ�����ӽ���Ĥ���ĵ�������������������������Ϊ______kg��
4SiHCl3(g)���ﵽƽ���H2��SiHCl3�����ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020 mol/L����H2ȫ����Դ��ͼ�����ӽ���Ĥ���ĵ�������������������������Ϊ______kg�� 
 ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣮
ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣮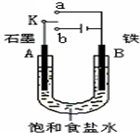 ��2008?��ݸģ�⣩ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣮
��2008?��ݸģ�⣩ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣮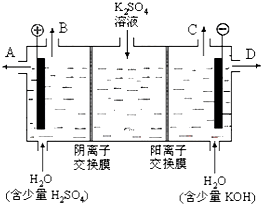 ����������������������أ�
����������������������أ�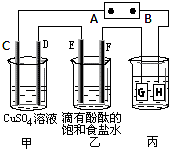 ij����С��ͬѧ����ͼװ�ý���ʵ�飬һ��ʱ�����C�缫������ͭ�������Իش��������⣮
ij����С��ͬѧ����ͼװ�ý���ʵ�飬һ��ʱ�����C�缫������ͭ�������Իش��������⣮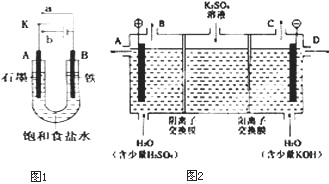 ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣺
ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣺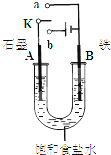 ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣮
ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣮