
���� ��1����ͭ�۵�ϡ�����еμ�����Ũ���ᣬ��Ӧ��������ͭ��NO��ˮ�������������������ã�
��2���������ӷ���ʽ�������㣻
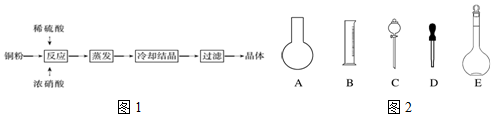
��3��������������Ϊ15%��480mLCuSO4��Һ����Ҫ��������500mL����ƿ���ձ�����ͷ�ιܣ���������������ƽ��ҩ�ף������ܶ�Ϊ1.07g/mL����������Ϊ15%��CuSO4��Һ500mL������ͭ�����ʵ������ټ�����ˮ����ͭ��������
��4����CuSO4•5H2O��NaHCO3��һ���ı�����ͬͶ�뵽150ml��ˮ�У����ҽ��裬��ȴ������ɫ�����������þ���Ļ�ѧ���ΪCux��OH��y��CO3��z•nH2O�������ľ����������������ӣ������Ƿ�ϴ�Ӹɾ�����ϴ��Һ���Ƿ�����������ɣ���ȡ3.640g���壬����������ϡ����ʹ������ȫ�ܽ⣬�ռ�����״���µ�����448.0mL����Ϊ0.02molӦΪ������̼���壬�ɴ˿ɼ����̼��������ʵ�����ȡ�������ľ��壬��������ȫ�ֽ⣬�õ�2.400g�������ӦΪ����ͭ���ɴ˼����������ͭԪ�ص����������ʵ��������ݵ���غ��ȷ���������������ʵ�����������������ͭԪ�ص�������̼�����������������������ȷ���ᾧˮ������������ȷ���������ɣ�
��5�������ܶȻ���������Һ������������Ũ�ȼ����c��Cu2+����
��0.1mol/L����ͭ��Һ��ͨ�����H2S���壬���������Ũ��Ϊ0.1mol/L��������Һ�ĵ����Լ���H+Ũ�ȣ�
��� �⣺��1����ͭ�۵�ϡ�����еμ�����Ũ���ᣬ��Ӧ��������ͭ��NO��ˮ�������������������ã�
�ʴ�Ϊ����������
��2����ͭ�۵�ϡ�����еμ�����Ũ���ᣬ��Ӧ��������ͭ�Ͷ���������ˮ���䷴Ӧ�����ӷ���ʽΪ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O����Ӧ������2molNO3-��8molH+��2mol�����ṩ2mol�����ӣ�������6mol�������������ṩ��������Ҫ������Ϊ3mol���������������������ȣ����ʵ���֮�ȣ�Ϊ2��3��
�ʴ�Ϊ��2��3��
��3��������������Ϊ15%��480mLCuSO4��Һ����Ҫ��������500mL����ƿ���ձ�����ͷ�ιܣ���������������ƽ��ҩ�ף�
�ܶ�Ϊ1.07g/mL����������Ϊ15%��CuSO4��Һ500mL�У�m��CuSO4��=500��1.07��15%g=80.25g������������ƽ��ȡCuSO4•5H2O������Ϊ$\frac{80.25��250}{160}g$=125.4g��
�ʴ�Ϊ��ABC���ձ�����������125.4��
��4���ٽ�CuSO4•5H2O��NaHCO3��һ���ı�����Ӧ�õ���ɫ���壬������ɫ����������ʱ�����Ӧ����������ӣ�����ͨ����������������ж��Ƿ�ϴ�Ӹɾ�������ķ�����ȡ���һ��ϴ��Һ�������Թ��У����������ữ��BaCl2��Һ�����а�ɫ������������δϴ������֮ϴ����
�������֪��n��CO32-��=n��CO2��=0.448L�M22.4mol•L-1=0.02 mol��
�������յõ��Ĺ�����CuO��
n��Cu2+��=n��CuO��=2.400 g/80 g•mol-1=0.03 mol��
���ݵ���غ㣺n��Cu2+����2=n��OH-��+n��CO32-����2��
����n��OH-��=n��Cu2+����2-n��CO32-����2=0.03 mol��2-0.02 mol��2=0.02 mol
n��H2O��=��3.640g-0.03mol��64g•mol-1-0.02mol��17g•mol-1-0.02mol��60g•mol-1��/18g•mol-1=0.01mol
����x��y��z��n=0.03mol��0.02mol��0.02mol��0.01mol=3��2��2��1������Ļ�ѧʽΪCu3��OH��2��CO3��2•H2O��Cu��OH��2•2CuCO3•H2O��
�ʴ�Ϊ��ȡ���һ��ϴ��Һ�������Թ��У����������ữ��BaCl2��Һ�����а�ɫ������������δϴ������֮ϴ����Cu3��OH��2��CO3��2•H2O��Cu��OH��2•2CuCO3•H2O��
��5���������Cu��OH��2���ܶȻ�����ȷ��pH=8ʱ��c��OH-��=10-6mol/L��Ksp[Cu��OH��2]=2.2��10-20����c��Cu2+��=$\frac{2.2��1{0}^{-20}}{��1��1{0}^{-6}��^{2}}$=2.2��10-8mol•L-1��
��0.1mol•L-1����ͭ��Һ��ͨ�����H2S���壬ʹCu2+��ȫ����ΪCuS����ʱ��Һ�е�����Ϊ���ᣬc��SO42-�����䣬Ϊ0.1mol•L-1���ɵ���غ��֪c��H+��Ϊ0.2mol•L-1��
�ʴ�Ϊ��2.2��10-8��0.2��
���� ���⿼�������ʵ��Ʊ�����Һ���ơ����ʻ�ѧʽ��ȷ�����ܶȻ���������֪ʶ����Ŀ�ѶȽϴ����ڿ���ѧ����ʵ��̽�������ͼ���������


 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ϊ��ɫ����������п��ܺ���SiO32- | |
| B�� | ����Ϊ����ɫ����������п��ܺ���FeBr3 | |
| C�� | ����Ϊ����ɫ����������п��ܺ���S2- | |
| D�� | ����Ϊ��ɫ�����������һ������FeCl3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��֬��� | B�� | ʯ���ѽ� | C�� | �������� | D�� | �ɱ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A | B | C | D | |
| ��Ʒ |  |  |  |  |
| ��Ҫ�ɷ� | Si3N4 | Al��OH��3 | Si | Fe2O3 |
| ��; | �������������� | ����ҩ | ���ά | Ϳ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ����ʽΪC9H11O5 | |
| B�� | ���Էֱ����Ҵ���������һ�������·�Ӧ���ҷ�Ӧ������ͬ | |
| C�� | ��ʹ������Ȼ�̼��Һ�����Ը��������Һ��ɫ������ɫԭ����ͬ | |
| D�� | ���б���������ͬ���칹����4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Al2��SO4��3��Һ�м��������ˮ��Al3++3OH-�TAl��OH��3�� | |
| B�� | ��Fe��OH��3�����м����������Һ��Fe��OH��3+3H+�TFe3++3H2O | |
| C�� | ��NaClO��Һ������������Һ��ϣ�ClO-+SO32-�TSO42-+Cl- | |
| D�� | ��ʯī���缫����Ȼ�þ��Һ��2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$H2��+Cl2��+2OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ԭ�ᱻ��Ϊ��������Ӫ���ء������й㷺��ɱ���������ܣ���ṹ��ʽ��ͼ��ʾ�������й���ԭ���˵������ȷ���ǣ�������
��ԭ�ᱻ��Ϊ��������Ӫ���ء������й㷺��ɱ���������ܣ���ṹ��ʽ��ͼ��ʾ�������й���ԭ���˵������ȷ���ǣ�������| A�� | ��ԭ������к���3�ֹ����� | |
| B�� | 1 mol��ԭ���������7molNaOH������Ӧ | |
| C�� | ��ԭ���ܷ���ȡ����Ӧ���ӳɷ�Ӧ����ȥ��Ӧ | |
| D�� | ��ԭ����ʹ���Ը��������Һ��Ũ��ˮ��ɫ����Ӧԭ��ͬ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com