
| A��1/3B2H6 (g) + O2 (g) ==1/3B2O3 (g) + H2O (g)��H =" -677.7" kJ��mol-1 |
| B��B2H6 (g) + 3O2 (g)="=" B2O3(s) + 3H2O (g)��H =" -2165" kJ��mol-1 |
| C��B2H6 (g) + 3O2 (g)="=" B2O3(s) + 3H2O (g)��H =" -2033" kJ��mol-1 |
| D��B2H6 (g) + 3O2 (g)== B2O3(s) + 3H2O��l����H =" -2165" kJ��mol-1 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


| A����NA�ĸ�����ת��ʱ���÷�Ӧ�ų�1300KJ������ |
| B����NA��ˮ����������ΪҺ��ʱ������1300KJ������ |
| C����2NA��̼�����õ��Ӷ�����ʱ���ų�1300KJ������ |
| D����8NA��̼�����õ�������ʱ���ų�1300KJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A���к��Ȧ�H����57.3 kJ��mol��1,, ��2H��(aq)��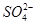 (aq)��Ba2��(aq)��2OH��(aq)===BaSO4(s)��2H2O(l)����H����114.6 kJ��mol��1 (aq)��Ba2��(aq)��2OH��(aq)===BaSO4(s)��2H2O(l)����H����114.6 kJ��mol��1 |
| B����0.5 molN2��1.5 mol H2���ܱ���������NH3(g)������19.3 kJ������ʽΪ�� N2(g)+3H2(g)  2NH3(g)��H="��38.6" kJ��mol��1 2NH3(g)��H="��38.6" kJ��mol��1 |
| C����״����H2(g)��F2(g) ==="2HF(g)" ��H����270kJ/mol�� |
| D����������4NH3(g)��5O2(g) ===4NO(g)��6H2O(g)��H����1025 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2��Q1��Q2��Q3�� | B��(Q1��Q2��Q3 ) | C��(Q1��Q2��Q3 ) | D��(3Q1��Q2��Q3 ) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
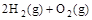 ��2H2O��g�� ��H2����483.6 kJ��
��2H2O��g�� ��H2����483.6 kJ��
| A��+131.3kJ��mol��1 | B����131.3kJ��mol��1 |
| C��+373.1kJ��mol��1 | D����373.1kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���� | B���ڢۢ� | C���� | D���٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2H2(g)+O2(g)=2H2O(l)��H= ��Q1kJ��mol-1�� 2H2(g)+O2(g)=2H2O(g)��H=��Q2kJ��mol-1 |
| B��S(g)+O2(g)=SO2(g)��H = ��Q1 kJ��mol-1�� S(s)+O2(g)=SO2(g)��H=��Q2kJ��mol-1 |
| C��C(s)+1/2O2(g)=CO(g)��H = ��Q1 kJ��mol-1�� C(s)+O2(g)=CO2(g)��H=��Q2kJ��mol-1 |
| D��H2(g)+Cl2(g)=2HCl(g)��H = ��Q1kJ��mol-1�� 1/2H2(g)+1/2Cl2(g)="HCl(g)" ��H= ��Q2kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2��g��=== ZnO(s)����H=" -348.3" kJ��mol-1��
O2��g��=== ZnO(s)����H=" -348.3" kJ��mol-1�� O2��g��=== Ag2O(s)����H=" -31.0" kJ��mol-1����Zn��s��+ Ag2O(s) === ZnO(s)+ 2Ag(s)�Ħ�H���ڣ� ��
O2��g��=== Ag2O(s)����H=" -31.0" kJ��mol-1����Zn��s��+ Ag2O(s) === ZnO(s)+ 2Ag(s)�Ħ�H���ڣ� ��| A��-317.3 kJ��mol-1 | B��-379.3 kJ��mol-1 | C��-332.8 kJ��mol-1 | D��317.3 kJ��mol-1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com