
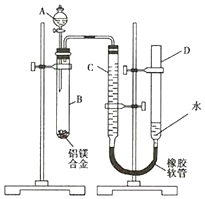
 ijѧϰС������ͼװ�òⶨþ���Ͻ�����������������
ijѧϰС������ͼװ�òⶨþ���Ͻ��������������������� Mg��NaOH����Ӧ����Al��Na��Ӧ����ʵ���֪��A��ΪNaOH��Һ��B�з���2Al+2NaOH+2H2O=2NaAlO2+3H2����C��DΪ����װ�ã���������������ɼ������������������������ԭ��������
��1��Mg��NaOH����Ӧ����Al��Na��Ӧ����ʵ���֪��A��ΪNaOH��Һ��
��2����þ�Ͻ�������������ʵ��ǰ���Ƚ���þ�Ͻ���ϡ�����н���Ƭ�̳�ȥ����������
��3���ڶ���ʱҪע������ȴ�����£���ʹD��CҺ����ƽ���������彫��ѹ����ɶ�������
��4��B�з�������Al���ķ�Ӧ����ƫ�����ƺ�������
��5��������Ӧ2Al+2NaOH+2H2O=2NaAlO2+3H2������������������ʵ��������������ʵ����õ���������������
��� �⣺Mg��NaOH����Ӧ����Al��Na��Ӧ����ʵ���֪��A��ΪNaOH��Һ��B�з���2Al+2NaOH+2H2O=2NaAlO2+3H2����C��DΪ����װ�ã�
��1��������������֪��AΪNaOH��Һ��
�ʴ�Ϊ��NaOH��Һ��
��2��ʵ��ǰ�Ƚ���þ�Ͻ���ϡ�����н���Ƭ�̣�Ŀ���dz�ȥ�Ͻ���������Ĥ��
�ʴ�Ϊ����ȥ�Ͻ���������Ĥ��
��3��Ҫȷ��������������뱣����Ͳ����������¶Ⱥ�ѹǿ��ȣ�����ڶ�ȡ��Ͳ����������֮ǰ��Ӧʹ�Թܺ���Ͳ�ڵ����嶼��ȴ�����£��ٵ�����Ͳ����Һ��߶�ʹ֮��ͬ��������ȷ��˳��Ϊ���ڢ٢ۣ�
�ʴ�Ϊ���ڢ٢ۣ�
��4��B�з�����Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2 -+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2 -+3H2����
��5����ʵ����������Ʒ������ΪWg���������������Ϊa L����״������
��ӦΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
2 3
n ��Al�� $\frac{aL}{22.4L/mol}$
n ��Al��=$\frac{2a}{3��22.4}$mol��
����Ʒ��Al����������=$\frac{\frac{2a}{3��22.4}mol��27g/mol}{Wg}$��100%=$\frac{45a}{56W}$��100%��
�ʴ�Ϊ��$\frac{45a}{56W}$��100%��
���� ���⿼�����ʺ����IJⶨ���漰Al�Ļ�ѧ���ʡ���ѧ��Ӧ����ʽ�ļ���ȣ��ۺ��Խ�ǿ��ע�ظ�Ƶ����Ŀ��飬��Ŀ�Ѷ��еȣ����ط�����ʵ������������Ŀ��飮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
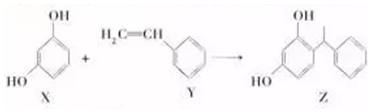
| A�� | lmolX����ˮ��Ӧ����3molBr2 | |
| B�� | X����Na2CO3��Һ��Ӧ | |
| C�� | Y���ܷ���ȡ����Ӧ��Ҳ�ܷ����ӳɷ�Ӧ | |
| D�� | ������KMnO4��Һ�ɼ���X��Y |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
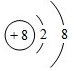 ��
�� ���������Ӽ����ۼ��Ļ�����Ļ�ѧʽΪNa2O2��
���������Ӽ����ۼ��Ļ�����Ļ�ѧʽΪNa2O2�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | CH3CH2OH | B�� | ˮ | C�� | ��֬ | D�� | �������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com