
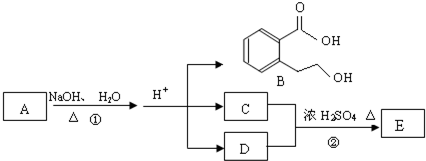
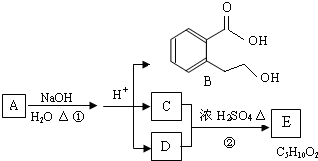
Ζ÷±π»ΓΥϋΟ«ΒΡ»ή“ΚΫχ–– Β―ιΘ§÷ς“Σ≤ΌΉςΦΑœ÷œσ»γœ¬ΘΚ
ΔΌœρAΒΡ»ή“Κ÷–Ά®»κΕΰ―θΜ·ΧΦΘ§‘ΌΦ”»κΤΖΚλ»ή“ΚΘ§Κλ…ΪΆ »ΞΓΘ
ΔΎΫΪBΚΆCΒΡ»ή“ΚΜλΚœΘ§…ζ≥…ΑΉ…Ϊ≥ΝΒμΘ§ΗΟ≥ΝΒμΩ…»ή”ΎE»ή“ΚΓΘ
ΔέΫΪBΚΆDΒΡ»ή“ΚΜλΚœΘ§…ζ≥…ΑΉ…Ϊ≥ΝΒμΘ§ΦΧ–χΦ”»κΙΐΝΩΒΡE»ή“ΚΘ§”–Τχ≈ί≤ζ…ζΒΡΆ§ ±ΜΙ”–ΑΉ…Ϊ≥ΝΒμ¥φ‘ΎΓΘ
ΔήAΚΆE»ή“ΚΒΡ―φ…ΪΖ¥”ΠΕΦ≥ ΜΤ…ΪΓΘ
(1)–¥≥ωœ¬Ν–Έο÷ ΒΡΜ·―ß ΫΘΚA_________ΓΔC_________ΓΔD_________ΓΘ
(2)–¥≥ωAΓΣE»ή“Κ÷–Θ§”κ¬ΝΖΔ…ζ÷ΟΜΜΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ_____________________________
(3)œρBΒΡ»ή“Κ÷–Θ§ΜΚ¬ΐΒΈ»κ…ΌΝΩœΓΒΡE»ή“ΚΘ§Τδ÷ς“ΣΒΡάκΉ”ΖΫ≥Χ ΫΈΣ__________________
(1)NaClO CaCl2ΜρCa(ClO)2 BaCl2ΜρBa(ClO)2
(2)2Al+6H+![]() 2Al3++3H2Γϋ
2Al3++3H2Γϋ
(3)H++![]()
![]()
![]()
ΓΨΫβΈωΓΩ¥”’ϊΧεΩΦ¬«Θ§”…≥θ≤Ϋ»ΖΕ®Θ§≤ΩΖ÷»ΖΕ®ΒΫΆξ»Ϊ»ΖΕ®άκΉ”ΒΡΖΫΖ®Θ§”…ΔΌ÷–œ÷œσΩ…“‘»ΖΕ®Κ§”–ClO-”…Δή»ΖΕ®Κ§”–Na+Θ§“ρ¥ΥAΈΣNaClOΘΜ”…BΡήΚΆCΘ§D…ζ≥…≥ΝΒμ»ΖΕ®B÷–Κ§”–![]() Θ§CΓΔD÷–Κ§Ca2+Θ§Ba2+ΘΜE÷–Κ§”–
Θ§CΓΔD÷–Κ§Ca2+Θ§Ba2+ΘΜE÷–Κ§”–![]() Θ§‘ΎΔέ÷–œ÷œσ”÷”–≥ΝΒμ…ζ≥…Θ§ΥΒΟς «BaSO4Θ§C÷–Κ§Ca2+Θ§D÷–Κ§Ba2+Θ§CΓΔDΈΣΩ…»ή–‘―ΈΘ§“ρ¥ΥCΈΣCaCl2ΜρCa(ClO)2Θ§DΈΣBaCl2ΜρBa(ClO)2ΓΘ
Θ§‘ΎΔέ÷–œ÷œσ”÷”–≥ΝΒμ…ζ≥…Θ§ΥΒΟς «BaSO4Θ§C÷–Κ§Ca2+Θ§D÷–Κ§Ba2+Θ§CΓΔDΈΣΩ…»ή–‘―ΈΘ§“ρ¥ΥCΈΣCaCl2ΜρCa(ClO)2Θ§DΈΣBaCl2ΜρBa(ClO)2ΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
| ≈®ΝρΥα |
| Γς |
| ≈®ΝρΥα |
| Γς |




≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
| ≈®ΝρΥα |
| Φ”»» |
| ≈®ΝρΥα |
| Φ”»» |


 –¥≥ωΥΡ’Ώ÷°“ΜΦ¥Ω…
–¥≥ωΥΡ’Ώ÷°“ΜΦ¥Ω… –¥≥ωΥΡ’Ώ÷°“ΜΦ¥Ω…
–¥≥ωΥΡ’Ώ÷°“ΜΦ¥Ω…| Β―ι±ύΚ≈ | CΈο÷ ΒΡΝΩ≈®Ε»Θ®mol?L-1Θ© | NaOHΈο÷ ΒΡΝΩ≈®Ε»Θ®mol?L-1Θ© | ΜλΚœ»ή“ΚΒΡpH |
| m | 0.1 | 0.1 | pH=9 |
| n | 0.2 | 0.1 | pHΘΦ7 |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ







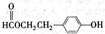
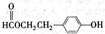
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ

| ≈®ΝρΥα |
| Γς |
| ≈®ΝρΥα |
| Γς |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
| Έο÷ ±ύΚ≈ | Έο÷ ΉΣΜ·ΙΊœΒ | A | D | E |
| ΔΌ |  |
Si | SiO2 | H2SiO3 |
| ΔΎ | N2 | NO2 | HNO3 | |
| Δέ | S | SO3 | H2SO4 | |
| Δή | Na | Na2O2 | NaOH |
| AΓΔΔΎΔέ | BΓΔΔΎΔή |
| CΓΔΔΌΔέΔή | DΓΔΔΌΔΎΔέΔή |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com