
 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2012?��ׯ��ģ��̼��̼�Ļ����������������������е�Ӧ�÷dz��㷺����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�
��2012?��ׯ��ģ��̼��̼�Ļ����������������������е�Ӧ�÷dz��㷺����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�| n(H2) | n(CO2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

 CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;����
CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;����
| ||
| ||
| ||
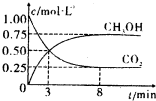
 CO+3H2
CO+3H2 CH3OH��g��+H2O��g����H=-49.0kJ/mol�����CO2��CH3OH��g����Ũ����ʱ��仯������ͼ��ʾ��
CH3OH��g��+H2O��g����H=-49.0kJ/mol�����CO2��CH3OH��g����Ũ����ʱ��仯������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com