
| �¶�/�� | 0 | 10 | 20 | 30 | 50 | 80 | 100 |
| �ܽ��(g/100gH20) | 74.4 | 81.9 | 91.8 | 106.8 | 315.1 | 525.8 | 535.7 |
 ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
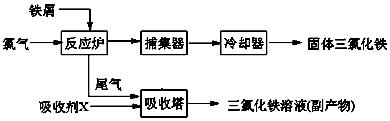
 ��
�� �������� ��
�������� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 |
| ��ҵ������Ʒ���� | ��ӦǰE�������� | ��Ӧ��E�������� |
| 6.4g | 51.9g | 54.1g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
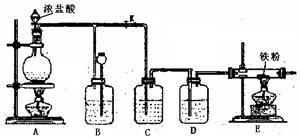
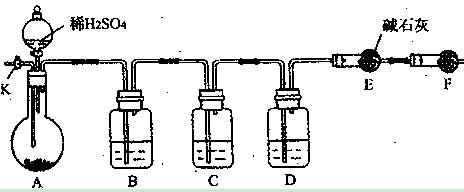
 ��4��E����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е������� ��B�������� ��
��4��E����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е������� ��B�������� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
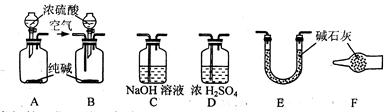
 Fe2O3+SO3��SO3��+14H2O���������������ʵ��װ������֤�̷����ȷֽ��Ƿ���������Ӧ��
Fe2O3+SO3��SO3��+14H2O���������������ʵ��װ������֤�̷����ȷֽ��Ƿ���������Ӧ��
| | ʵ���¼ | ����������� |
| ���� | Ӳ�ʹ�����ɫ��Ϊ����ɫ�� | �������� |
| �����B�о����ɫ����ɫ�� | ��������H2O | |
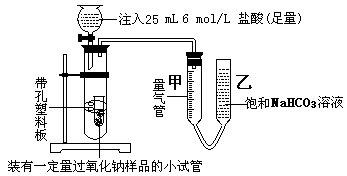
| ���� | ��ȡ�̷���Ʒ16.68g�� | ��Ӧ���̷�0.06mol |
| �����E�������2.24g�� | ����SO2 mol | |
| C�����ɰ�ɫ����������Ϊ4.66g�� | ����DO3 0.02mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ��Ŀ�� | ʵ�鲽�輰���� |
| A | �����������������Ƿ���� |  |
| B | ����ij±�����Ƿ����ȴ��� |  |
| C | ֤������������H2O2�����Ա�I2ǿ |  |
| D | ��ȥ�Ȼ�����Һ�е����������� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������C2H5Cl�е���Ԫ�أ��Ƚ�C2H5Cl��NaOH��Һ��Ϻ���ȣ��ټ������ữ |
| B�����KMnO4��Һ�����������������ὫKMnO4��Һ�ữ |
| C��������Һ���Ƿ���Fe2+���Ƚ���Һ�������ữ���ټ���KSCN��Һ |
| D��������Һ���Ƿ���SO42�����Ƚ���Һ�������������ữ���ټ���BaCl2��Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com