
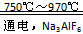 4Al+3O2��
4Al+3O2�� 
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ͨ�� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
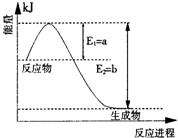
| ���� |
| ���¸�ѹ |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ��ͨ�к����ظ߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��14�֣��绯ѧԭ�����ִ�����������������ҪӦ�á�
��ȼ�ϵ����Ŀǰ��չ��ͷǿ����������ɫ������ء�ij��������ȼ�ϵ����H2Ϊȼ�ϣ�O2Ϊ��������H2SO4��ҺΪ���Һ����д���õ�ص��ܷ�Ӧ����ʽ�� ��
�������������Խ��Al��AgO��ؿ�����ˮ�¶�����Դ���õ�ط�Ӧ��ԭ��Ϊ��
Al+3AgO+2NaOH=2NaAlO2+3Ag+H2O��
�ٸõ�ط�Ӧ�ĸ����缫��Ӧʽ ��
��ͼ�и�ĤΪ�����ӽ���Ĥ����Һ��OH��ͨ���� ���ƶ���ѡ�������������
��Al��AgO�����Ϊ��Դ��ʹ��Pt�缫���500mL����NaCl��Һ�����һ��ʱ��ָ������£�������ҺpH=13������NaCl��Һ�����ҵ��ǰ��������䣩��
��ʹ��Pt�缫���NaCl��Һ�����ӷ���ʽ ��
�������зų��������������״���£�Ϊ L��
������ص�Ч��Ϊ50%���ù��������Ľ�����������Ϊ g��
���õ�ⷨ��ȡþʱ����ԭ���Ȼ�þ����ˮʱ���ڵ���¶��£�ԭ�ϻ��γ�Mg(OH)Cl�����������룺Mg(OH)C1 �� Mg(OH)�� �� C1һ��
���ʱ������������������þ�ۻ�Ĥ����ʱ�����ķ�ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ��ͨ�к����ظ߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��14�֣��绯ѧԭ�����ִ�����������������ҪӦ�á�
��ȼ�ϵ����Ŀǰ��չ��ͷǿ����������ɫ������ء�ij��������ȼ�ϵ����H2Ϊȼ�ϣ�O2Ϊ��������H2SO4��ҺΪ���Һ����д���õ�ص��ܷ�Ӧ����ʽ�� ��
�������������Խ��Al��AgO��ؿ�����ˮ�¶�����Դ���õ�ط�Ӧ��ԭ��Ϊ��
Al+3AgO+2NaOH=2NaAlO2+3Ag+H2O��

�ٸõ�ط�Ӧ�ĸ����缫��Ӧʽ ��
��ͼ�и�ĤΪ�����ӽ���Ĥ����Һ��OH��ͨ���� ���ƶ���ѡ�������������
��Al��AgO�����Ϊ��Դ��ʹ��Pt�缫���500mL����NaCl��Һ�����һ��ʱ��ָ������£�������ҺpH=13������NaCl��Һ�����ҵ��ǰ��������䣩��
��ʹ��Pt�缫���NaCl��Һ�����ӷ���ʽ ��
�������зų��������������״���£�Ϊ L��
������ص�Ч��Ϊ50%���ù��������Ľ�����������Ϊ g��
���õ�ⷨ��ȡþʱ����ԭ���Ȼ�þ����ˮʱ���ڵ���¶��£�ԭ�ϻ��γ�Mg(OH)Cl�����������룺Mg(OH)C1 �� Mg(OH)�� �� C1һ��
���ʱ������������������þ�ۻ�Ĥ����ʱ�����ķ�ӦʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com