
£Ø12·Ö£©
ŌŚŅ»¶ØĢõ¼žĻĀ£¬ĻĀĮŠø÷ĪļÖŹæÉ·¢ÉśČēĻĀĶ¼ĖłŹ¾µÄ±ä»Æ£ŗ
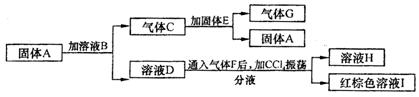
ĮķÖŖ£ŗA”¢D”¢E”¢HµÄŃęÉ«·“Ó¦¾ł³Ź»ĘÉ«£»GÄÜŹ¹“ų»šŠĒµÄľĢõø“Č¼£»ČÜŅŗBÖŠ¼ÓAgNO3ČÜŅŗ²śÉśµ»ĘÉ«³Įµķ£»ČÜŅŗHÖŠ¼ÓAgNO3ČÜŅŗ²śÉś°×É«³Įµķ”£
£Ø1£©Š“³öA”¢BµÄ»ÆѧŹ½£ŗA__________,B______________”£
£Ø2£© EÖŠ“ęŌŚµÄ»Æѧ¼üĄąŠĶĪŖ£ŗ______________”¢_________________”£
£Ø3£© Š“³öĘųĢåCŗĶ¹ĢĢåE·“Ó¦µÄ»Æѧ·½³ĢŹ½_______________________________”£
£Ø4£© Š“³öČÜŅŗDŗĶĘųĢåF·“Ó¦µÄĄė×Ó·½³ĢŹ½_______________________________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ¼×”¢ŅŅ”¢±ū”¢¶””¢ĪģŹĒĪåÖÖ¶ĢÖÜĘŚŌŖĖŲ£¬ĘäŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬ĖüĆĒæÉŅŌ×é³ÉĻĀĮŠæņĶ¼ĖłÓŠĪļÖŹ£®¼×ÓėŅŅ”¢±ūÓėŅŅ¾łæÉŠĪ³ÉŌ×ÓøöŹż±ČĪŖ2£ŗ1ŗĶ1£ŗ1µÄ»ÆŗĻĪļ£¬¶”ŌŖĖŲŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒĘäµē×Ó²ćŹżµÄ2±¶£¬æņĶ¼ÖŠČÜŅŗMĪŖ»ĘĀĢÉ«£®ŌŚŅ»¶ØĢõ¼žĻĀ£¬ĻĀĮŠø÷ĪļÖŹæÉ·¢ÉśČēĶ¼ĖłŹ¾µÄ±ä»Æ£®ŹŌ»Ų“š£ŗ
¼×”¢ŅŅ”¢±ū”¢¶””¢ĪģŹĒĪåÖÖ¶ĢÖÜĘŚŌŖĖŲ£¬ĘäŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬ĖüĆĒæÉŅŌ×é³ÉĻĀĮŠæņĶ¼ĖłÓŠĪļÖŹ£®¼×ÓėŅŅ”¢±ūÓėŅŅ¾łæÉŠĪ³ÉŌ×ÓøöŹż±ČĪŖ2£ŗ1ŗĶ1£ŗ1µÄ»ÆŗĻĪļ£¬¶”ŌŖĖŲŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒĘäµē×Ó²ćŹżµÄ2±¶£¬æņĶ¼ÖŠČÜŅŗMĪŖ»ĘĀĢÉ«£®ŌŚŅ»¶ØĢõ¼žĻĀ£¬ĻĀĮŠø÷ĪļÖŹæÉ·¢ÉśČēĶ¼ĖłŹ¾µÄ±ä»Æ£®ŹŌ»Ų“š£ŗ



²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ





²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗž±±Ź”ĪäŗŗŹŠĖÄŠ£ø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©¼×”¢ŅŅ”¢±ū”¢¶”ĖÄÖÖ¶ĢÖÜĘŚŌŖĖŲæÉŅŌ×é³ÉĻĀĮŠæņĶ¼ÖŠ³żBr2ŗĶLŅŌĶāµÄĪļÖŹ£¬ĘäŌ×ÓŠņŹżŅĄ“ĪŌö“󔣼×ŗĶŅŅæÉŠĪ³É³£¼ūŅŗĢ¬»ÆŗĻĪļK£¬¹ĢĢåAÖŠŗ¬ÓŠ±ūŌŖĖŲµÄÕżŅ»¼ŪŃōĄė×Ó£¬Ęäµē×Ó²ć½į¹¹ÓėÄŹŌ×ÓĻąĶ¬£¬¶”ŌŖĖŲŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒĘäµē×Ó²ćŹżµÄ2±¶”£ŌŚŅ»¶ØĢõ¼žĻĀ£¬ĻĀĮŠø÷ĪļÖŹæÉ·¢ÉśČēĶ¼ĖłŹ¾µÄ±ä»Æ£Ø·“Ó¦ÖŠÉś³ÉµÄĖ®Ć»ÓŠŠ“³ö£©£ŗ
ŹŌ»Ų“š£ŗ
(1)AµÄµē×ÓŹ½ĪŖ £»ŅŅŌŖĖŲµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ__________________”£
¢ĘÓÉĖŁĀŹĄķĀŪŌ¤²ā¹¤ŅµÉĻÓÉFÖĘČ”HµÄ·“Ó¦Ģõ¼žĪŖ__________________”£
¢ĒČÜŅŗBµÄpHÖµŠ”ÓŚ7£¬ĘäŌŅņŹĒ__________________ ”£
¢Č·“Ó¦£ØI£©µÄ»Æѧ·½³ĢŹ½ĪŖ __________________ ”£
¢É·“Ó¦£ØII£©µÄĄė×Ó·½³ĢŹ½ĪŖ __________________ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com