
��12�֣���1��CH3����CH3����CH3��������Ҫ���л���Ӧ�м��壬�й����ǵ�˵����ȷ���� ��
A�����Ǿ��ɼ���ȥ��һ����ԭ������
B�����ǻ�Ϊ�ȵ����壬̼ԭ�Ӿ���ȡsp2�ӻ�
C��CH3����NH3��H3O+��Ϊ�ȵ����壬���ι��;�Ϊ������
D��CH3���е�̼ԭ�Ӳ�ȡsp2�ӻ�������ԭ�Ӿ�����
E������CH3����һ��CH3����CH3����Ͼ��ɵõ�CH3CH3
��2��п��һ����Ҫ�Ľ�����п���仯�������Ź㷺��Ӧ�á�
��ָ��п�����ڱ��е�λ�ã� ���ڣ� �壬 ����
����������п[CH2OH��CHOH��4COO]2Zn��Ŀǰ�г������еIJ�п����д��Zn2+��̬�����Ų�ʽ �������Ƿ�����̼ԭ���ӻ���ʽ�� ��
��Zn2+����NH3�γ�������[Zn��NH3��4]2+����λ��NH3�������� ������Է��ӡ��Ǽ��Է��ӡ�������[Zn��NH3��4]2+�У�Zn2+λ�������������ģ�Nλ����������Ķ��㣬��������ͼ�б�ʾ[Zn��NH3��4]2+��Zn2+��N֮��Ļ�ѧ����


������ͼ��ʾп��ij�ǽ���Ԫ��X�γɵĻ����ᄃ��������Zn��Xͨ�����ۼ���ϣ��û�����Ļ�ѧʽΪ ���û�����ľ����۵�ȸɱ��ߵö࣬ԭ���� ��
 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� |

| KMnO4 |
| H+ |










 ��
��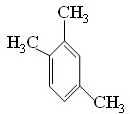
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
 ��������____________��
������Ϊ��____________�� �鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com