
��ˮ��Դ�ḻ����ˮ����Ҫ����
![]() �����ӡ��������ú�ˮ��Դ�ͱ����������ҹ��ɳ�����չ����Ҫ��֤��
�����ӡ��������ú�ˮ��Դ�ͱ����������ҹ��ɳ�����չ����Ҫ��֤��
��������ȼú�ŷŵ�![]() �����һϵ�л�������̬���⡣���ú�ˮ������һ����Ч�ķ������乤����������ͼ��ʾ��
�����һϵ�л�������̬���⡣���ú�ˮ������һ����Ч�ķ������乤����������ͼ��ʾ��
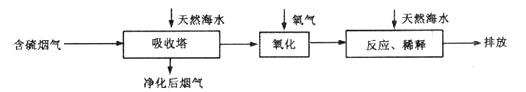
��1����Ȼ��ˮ�Լ��Ե�ԭ���ǣ������ӷ���ʽ��ʾ���� ��
��2����Ȼ��ˮ�����˺���������Ҫ��![]() ���������������䷴Ӧ�Ļ�ѧ����ʽ��__________________________��������ġ���ˮ����Ҫ�ô�������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ����___________________________��
���������������䷴Ӧ�Ļ�ѧ����ʽ��__________________________��������ġ���ˮ����Ҫ�ô�������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ����___________________________��
���ؽ������ӶԺ������������������Ⱦ��ij��������ˮ��pH=2.0���ѡ�1g��mL-1���к���![]() ���ؽ������ӣ���Ũ�ȸ�ԼΪ0.01mol��L-1���ŷ�ǰ���ó�������ȥ���������ӣ������й��������£�
���ؽ������ӣ���Ũ�ȸ�ԼΪ0.01mol��L-1���ŷ�ǰ���ó�������ȥ���������ӣ������й��������£�
| ���ܵ���� |
|
|
|
|
|
|
|
| 8.3��10-17 | 5.6��10-8 | 6.3��10-50 | 7.1��10-9 | 1.2��10-15 | 3.4��10-28 |
��3������Ϊ����ˮ��Ͷ��_________������ĸ��ţ�������Ч����á�
A��![]() B��
B�� ![]() C��
C��![]() D��
D��![]()
��4���������ʯ�Ҵ���������ˮ��ʹ��Һ��pH=8.0��������ķ�ˮ��![]() =_______��
=_______��
��5�������ʳ�δ�����ֻ��![]() �ķ�ˮ����ô�����ķ�ˮ��
�ķ�ˮ����ô�����ķ�ˮ��![]() ����������Ϊ0.117%��������Ҫ���ŷű�Ϊ
����������Ϊ0.117%��������Ҫ���ŷű�Ϊ![]() ����1.0��l0-8mol��L-1���ʸù���������ķ�ˮ��
����1.0��l0-8mol��L-1���ʸù���������ķ�ˮ��![]() =___���Ƿ�����ŷű�_____ ����ǡ�����
=___���Ƿ�����ŷű�_____ ����ǡ�����
��֪![]() =1.8��l0-10
=1.8��l0-10
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡ��������·ʵ����ѧ����ģ�⿼�������ۺϻ�ѧ�Ծ����������� ���ͣ������
(15��)��ѧ����������Ĺؼ�����ѧΪ����������������ṩ�����ʱ�֤��
(1)���ʱ���öƲ������������������ ��Ϊ��ʹ�Ʋ��Ⱦ��ȡ��⻬���ܡ���Ƽ��ĸ�����ǿ����������Һ������Ũ���⣬ͨ�������Բ�ȡ�Ĵ�ʩ��
(2)±ˮ���̺��ŷḻ��þ��Դ����ת����ɻ��MgCl2�ֲ�Ʒ����±ˮ����ȡþ�IJ���Ϊ��
a�������ߴ������ڵı������ճ�ʯ�ң�����ʯ���Ƴ�ʯ���飻
b����ʯ������뵽��ˮ�������о����˵õ�Mg(OH)2������
c.��Mg(OH)2�����м�������õ�MgCl2��Һ���پ������ᾧ�õ�MgCl2��6H2O��
d����MgCl2��6H2O��һ�������¼��ȵõ���ˮMgCl2��
e��������ڵ��Ȼ�þ�ɵõ�Mg��
�ٲ���d�еġ�һ��������ָ���� ��Ŀ���� ��
��������ȡþ��������,Ϊ�˽��ͳɱ���������Ⱦ�����Բ�ȡ�ܶ��ʩ����д������һ��
����ͬѧ��Ϊ������b��ɼ���Mg(0H)2�õ�Mg0���ٵ�����ڵ�MgO�ƽ���þ�������ɼ�ʵ�鲽�裬��ͬ���ͬѧ���뷨��?Ϊʲô?
��
(3)���Ǻ˷�Ӧ����Ҫ��ȼ�ϣ��Ѿ����Ƴɹ�һ���������ӽ�����֬����ר��������ˮ�е�U4+��������������Ԫ�ء��䷴Ӧԭ��Ϊ (��֬��HR����)���������ӽ���������ӽ���Ĥ���ᴦ�������������õ����˵���Һ���䷴Ӧԭ�� Ϊ ��
(4)��˾ƥ��( )�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ�� ���˷�Ӧ���������� ��
)�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ�� ���˷�Ӧ���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ�����и���ģ�⿼�������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
(15��)��ѧ����������Ĺؼ�����ѧΪ����������������ṩ�����ʱ�֤��
(1)���ʱ���öƲ������������������ ��Ϊ��ʹ�Ʋ��Ⱦ��ȡ��⻬���ܡ���Ƽ��ĸ�����ǿ����������Һ������Ũ���⣬ͨ�������Բ�ȡ�Ĵ�ʩ��
(2)±ˮ���̺��ŷḻ��þ��Դ����ת����ɻ��MgCl2�ֲ�Ʒ����±ˮ����ȡþ�IJ���Ϊ��
a�������ߴ������ڵı������ճ�ʯ�ң�����ʯ���Ƴ�ʯ���飻
b����ʯ������뵽��ˮ�������о����˵õ�Mg(OH)2������
c.��Mg(OH)2�����м�������õ�MgCl2��Һ���پ������ᾧ�õ�MgCl2��6H2O��
d����MgCl2��6H2O��һ�������¼��ȵõ���ˮMgCl2��
e��������ڵ��Ȼ�þ�ɵõ�Mg��
�ٲ���d�еġ�һ��������ָ���� ��Ŀ���� ��
��������ȡþ��������,Ϊ�˽��ͳɱ���������Ⱦ�����Բ�ȡ�ܶ��ʩ����д������һ��
����ͬѧ��Ϊ������b��ɼ���Mg(0H)2�õ�Mg0���ٵ�����ڵ�MgO�ƽ���þ�������ɼ�ʵ�鲽�裬��ͬ���ͬѧ���뷨��?Ϊʲô?
��
(3)���Ǻ˷�Ӧ����Ҫ��ȼ�ϣ��Ѿ����Ƴɹ�һ���������ӽ�����֬����ר��������ˮ�е�U4+��������������Ԫ�ء��䷴Ӧԭ��Ϊ (��֬��HR����)���������ӽ���������ӽ���Ĥ���ᴦ�������������õ����˵���Һ���䷴Ӧԭ�� Ϊ ��
(4)��˾ƥ��( )�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��
���˷�Ӧ����������
��
)�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��
���˷�Ӧ����������
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡģ���� ���ͣ������
2010�����˻����ҹ����ݳɹ��ٰ죬�������˻������˻�������
(1)�������˻��桰���������ñ���(C3H8)��ȼ�ϣ�ȼ�պ�IJ���Ϊˮ�Ͷ�����̼����298Kʱ��1mol������ȫȼ������CO2��Һ̬ˮ�ų�2221. 5 kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ__________________��
��֪����298Kʱ��
C3H8(g)=C3H6(g) +H2(g)����H= +124.2 kJ/mol��
H2(g)+O2(g)=H2O(l); ��H= -285.8 kJ/mol��
��l mol C3H8��ȫȼ������CO2��Һ̬ˮʱ�ų���������___________kJ��
(2)������һ�������ĺ������У���ˮ��Դ�dz��ḻ��
�ٺ�����������Ϊ����������Ϊ��������ˮΪ�������Һ�������е�����������Ӧ��������������ܷ�ӦΪ��4Al+3O2+6H2O=4Al(OH)3������˵����ȷ����_________����д�����ĸ����
a����ع���ʱ�����������缫�ص������缫
b�����缫������״�ȿ�״������O2�ŵ�
c����ˮ�е�OH-�����缫�����ƶ�
�������£��ö��Ե缫���200mL l.5 mol/Lʳ��ˮ�� ���2minʱ���������ռ���448 mL���壨��״���£���д���õ�ⷴӦ�����ӷ���ʽ_________________��������ǰ����Һ��������䣬��������Һ��pHΪ_____��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com