
| C | D | I |
��ʼ���/mol | 6 | 4 | 0 |
ijʱ�����/mol | 3 | 3 | 2 |
����A��B��Ӧ��������E��F��G������C��D��Ӧ��������I��ij�¶��¸÷�Ӧ��ʼ��ijʱ�̵ķ�Ӧ�����������ϱ�����ʾ������д���пհף�
��1������H�ķ���ʽ�ǣߣߣߣߣߣߡ�
��2����Ӧ�ٵĻ�ѧ����ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��3����Ӧ�ڵĻ�ѧ����ʽ����ע����Ӧ�������ǣߣߣߣߣߣߣߣߣߡ�
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


 NH4++NH2-
NH4++NH2- NH4++NH2-
NH4++NH2-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
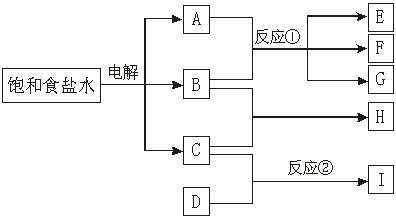
 ��ͼÿһ�����е���ĸ����һ�ַ�Ӧ��������
��ͼÿһ�����е���ĸ����һ�ַ�Ӧ��������
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
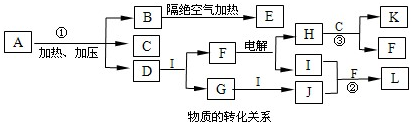
| ���¸�ѹ |
| ||
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
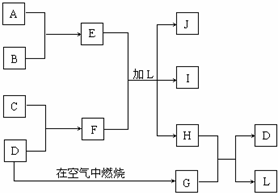
| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣���ͼÿһ�����е���ĸ����һ�ַ�Ӧ��������
����J�Ǻ�A����Ԫ�صĽ�״��ɫ����������E�백ˮ��Ӧ��ȡ��IΪ�Ȼ�����Һ��D�ǵ���ɫ���嵥�ʡ�����д���пհף�
��1��F�Ļ�ѧʽΪ��_________________��
��2��L�Ļ�ѧʽΪ��______________��
��3��д��J���ȷֽⷴӦ�Ļ�ѧ����ʽ��____________________��
��4��H��G֮�䷴Ӧ�Ļ�ѧ����ʽΪ��________________________��
(5) E�백ˮ��Ӧ�����ӷ���ʽ_______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com