
(��16��) ���������ʵ���Ũ��Ϊa mol/L�ı�����ȥ�ⶨV mL NaOH��Һ�����ʵ���Ũ�ȣ�����д���пհף�
�� ��ʽ�ζ���������ˮϴ����Ӧ�ý��еIJ�����___��___��
 �� ��ͼ����ʽ�ζ�����Һ���ڵζ�ǰ��Ķ�����
�� ��ͼ����ʽ�ζ�����Һ���ڵζ�ǰ��Ķ�����
�����йط��ű�ʾ�ô���NaOH��Һ�����ʵ���Ũ�ȣ�c (NaOH) = ___��___ mol/L��
�� ���ڵζ�ǰ�ζ��ܼ��첿���������ݣ��ζ���ζ��ܼ��첿��������ʧ����ⶨ��NaOH���ʵ���Ũ�Ȼ�ƫ___��___��
����֪�����к���N2��O2��CO2��H2S�����塣���ж����еζ��������յ㡢�����������������ԭ�������й����ӷ���ʽ��ʾ��
�� �Է�̪Ϊָʾ������Һ�ζ���Һ���� �� Ϊ�յ㡣30s������ɫ��ԭ�� �� ��
�� �Ե���Ϊָʾ������Na2S2O3�ζ�I2(2S2O32��+I2 �� S4O62��+2I��)�� �� Ϊ�յ㣬Լ5min����Һ����ɫ��ԭ�� �� ��
 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֡�����ijѧ����Ƶ��ô���������ȡ����HNO3��Һ��ʵ��װ�ã�����ͼ��ʾ��
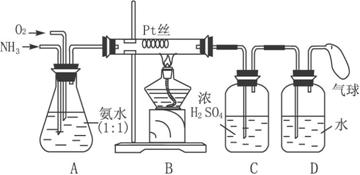
��ش��������⡣
(1)ʵ�����Ʊ�NH3�����з�������ѡ�õ���________(�����)
�ٹ�̬�Ȼ������ʯ�һ�ϼ���
�ڹ�̬�Ȼ�識��ȷֽ�
����ʯ���еμ�Ũ��ˮ
���Ȼ����Һ������������Һ����
(2)װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ______________����ʵ������У�����Pt˿���Ⱥ���ȥ�ƾ��ƣ�����Pt˿���������ֺ��ȣ��ɴ˿��жϸ÷�Ӧ��_____________________��
(3)װ��C��������__________��װ��C�е�������__________��Ϊȷ��D�о����ܶ�����HNO3����ͨ��O2��NH3�������Ӧ����__________��
(4)���ڸ����´�����ʱ���и���Ӧ������4NH3+3O2![]() 2N2+6H2O��������װ���в�����������������D����1.0 mol��L-1��HNO3��Һ150 mL���������ռ��Ļ���������Ϊ400 mL(��״��)������NO2��O2��N2�������Ϊ2��2��1����������NO�İ�ռ���������������Ϊ_________��
2N2+6H2O��������װ���в�����������������D����1.0 mol��L-1��HNO3��Һ150 mL���������ռ��Ļ���������Ϊ400 mL(��״��)������NO2��O2��N2�������Ϊ2��2��1����������NO�İ�ռ���������������Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ��ˮ�ж��и���ģ�⣨5�£����Ի�ѧ�Ծ����������� ���ͣ������
��ÿ��2�֣���16�֣�������Ԫ��X��Y��Z��W��ԭ���������������³�ѹ�£�ֻ��W�ĵ���Ϊ���塣���ǵ�����������Ӧ��ˮ��������Ϊ�ס��ҡ����������ס��ҡ�������ѧ��ѧ�еij������ʣ�����ֻ����������ˮ�����ܺͼס�����Ӧ�õ�������Һ������������Ϣ��д���пհף�
�Ż���W��ԭ�ӽṹʾ��ͼ_________________________________________��
�ƽ��Һͼס����ֱ�Ӧ��õ�����Һ��ϣ��۲쵽��������___________________
___________________ ������Һ���ʱ��������Ӧ�����ӷ���ʽΪ____________________________________________________��
��������ʵ��֤��Z��W�ǽ�����ǿ�����ǣ�ѡ����ţ�__________________________��
| A�����ʵ��۵㣺Z��W2 |
| B�����ԣ������� |
| C������Һ�У�W2��H2Z��2HW��Z |
| D���ȶ��ԣ�HW��H2Z |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ�Ű���ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
����16�֣���1������ͭ������ܽ������ܽ���̽�����ʵ���ҳ�����ˮ�����Լӿ��ܽ����ʣ���������������ǣ����Ҫ˵��ԭ��______________________����β�������ˮ���Ƴ�����Ľ�Ũ��CuSO4��Һ____________________��
��2��ϡNa2S��Һ��һ�ֳ�������ζ������AlCl3��Һ��������ζ�Ӿ磬�����ӷ���ʽ��ʾ��ζ�Ӿ�����������Ļ�ѧ��Ӧ______________________________________________
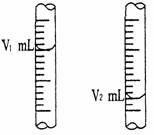
��ij�ռ���Ʒ�к����������������õĿ��������ʣ�Ϊ�˲ⶨ�䴿�ȣ��������µζ�������
| A������Һת����250 mL����ƿ�У���ˮ���̶��ߣ� |
| B������Һ��(���ʽ�ζ���)��ȡ25.00 mL�ռ���Һ����ƿ�в��Ӽ��μ�����ָʾ���� |
| C������ƽ��ȷ��ȡ�ռ���Ʒw g�����ձ��м�����ˮ�ܽ⣻ |
| D�������ʵ���Ũ��Ϊm mol?L��1�ı�H2SO4��Һװ����ʽ�ζ��ܣ�����Һ�棬���¿�ʼ�̶���ΪV1 mL�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��ˮ�и���ģ�⣨5�£����Ի�ѧ�Ծ��������棩 ���ͣ������
��ÿ��2�֣���16�֣�������Ԫ��X��Y��Z��W��ԭ���������������³�ѹ�£�ֻ��W�ĵ���Ϊ���塣���ǵ�����������Ӧ��ˮ��������Ϊ�ס��ҡ����������ס��ҡ�������ѧ��ѧ�еij������ʣ�����ֻ����������ˮ�����ܺͼס�����Ӧ�õ�������Һ������������Ϣ��д���пհף�
�Ż���W��ԭ�ӽṹʾ��ͼ_________________________________________��
�ƽ��Һͼס����ֱ�Ӧ��õ�����Һ��ϣ��۲쵽��������___________________
___________________ ������Һ���ʱ��������Ӧ�����ӷ���ʽΪ____________________________________________________��
��������ʵ��֤��Z��W�ǽ�����ǿ�����ǣ�ѡ����ţ�__________________________��
A.���ʵ��۵㣺Z��W2 B.���ԣ�������
C.����Һ�У�W2��H2Z��2HW��Z
D.�ȶ��ԣ�HW��H2Z E.�⻯��ˮ��Һ�����ԣ�HW��H2Z
F.�ܽ��ԣ�������
����Y���ʺ���������õĽ������缫���õ������Ӳ������Һ�й���ԭ��أ���ԭ��ظ����ĵ缫��ӦʽΪ_______________________________________________ ��
�ɹ�ҵ����XWΪԭ�Ͽ��Խ��������������W2��������Ҫ��Ʒ��д����ҵ����XWΪԭ��������W2�Ļ�ѧ����ʽ_______________________________________________
_______________________________________________����Ҫ����80.0 kg�����ʣ�������ҪXW______________kg��ͬʱ�ɵ�W2_____________m3���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com