
����Ŀ����ͨ���������̻��շϾ�����ӵ����������(LiCoO2��������Al��Fe)�е��ܺ�ﮡ�
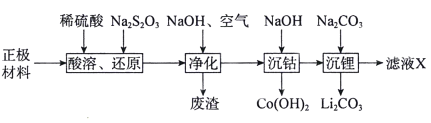
�ش��������⣺
(1)�����ܡ���ԭ��������S2O32-ת��ΪSO42-��LiCoO2���뷴Ӧ�����ӷ���ʽΪ____________________________________��
(2)������������Ҫ�ɷ�Ϊ___________________��
(3)�����ܡ������У�����Һ��pH=10ʱ��c(Co2+)=______mol�� L-1(��֪������KSP[Co(OH)2]=1.58��10-15)��
(4)�ڿ����м���Co(OH)2��������������¶ȵı仯��ͼ��ʾ��

290��ʱ����ȫ��ˮ��ΪCo2O3����Ӧ�Ļ�ѧ����ʽΪ_______________��500��ʱ����Ҫ����Ϊ_____________(�ѧʽ)����1000��ʱ�ķֽ����1mol��2.2mol Na2O(�Թ���)�ڳ�벷�չ��й��ȣ��������ʺ�ɫ�ľ��壬�þ����������Ϊ�������νṹ������Ļ�ѧʽΪ__________________��
(5)����ҺX��������Ҫ��������_______________(�ѧʽ)��
���𰸡�S2O32-+8LiCoO2+22H+=2SO42-+8Li++8Co2++11H2O Al(OH)3��Fe(OH)3 1.58��10-7 4Co(OH)2+O2![]() 2Co2O3+4H2O Co3O4 Na4CoO3 Na2SO4
2Co2O3+4H2O Co3O4 Na4CoO3 Na2SO4
��������
�������������Ҫ����LiCoO2������Al��Fe�ȣ�����ϡH2SO4�ܽ���������ܽ���������������������������Na2S2O3��S2O32-��������SO42-�����ɵ���Һ�к�������ﮡ������ܡ������ƣ���������������Һ��ͨ�����������������Ϊ�����ӣ��γ�������������������������������Һ�м��������������Ƶ�����ҺpH�������ܣ����˵õ��������ܣ���Һ�м���̼���Ƶ�����ҺpH������������γ�̼��ﮣ��õ���ҺX����Ҫ����Ϊ�����ơ�
��1���������Ϻ���LiCoO2������Al��Fe�ȣ�����ϡH2SO4��Na2S2O3��S2O32-��������SO42-�����л�ԭ�ԣ�����������ֻ��LiCoO2���������ԣ���Na2S2O3��Ӧ����CoSO4����Ӧ��ѧ����ʽΪ��8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O�������ӷ�Ӧ����ʽΪ��S2O32-+8LiCoO2+22H+=2SO42-+8Li++8Co2++11H2O���ʴ�Ϊ��S2O32-+8LiCoO2+22H+=2SO42-+8Li++8Co2++11H2O��
��2����������������Һ��ͨ�����������������Ϊ�����ӣ����γ��������������������ij��������ԡ�����������Ҫ�ɷ�Ϊ��Al(OH)3��Fe(OH)3���ʴ�Ϊ��Al(OH)3��Fe(OH)3��
��3������Һ��pH=10ʱ����Һ��c��H+��=10-10mol/L��c��OH-��=10-4mol/L����Ksp[Co(OH)2]=c��Co2+����c2��OH-��=1.58��10-15��c��Co2+��=1.58��10-7mol��L-1���ʴ�Ϊ��1.58��10-7��
��4�����������غ㶨�ɣ��ڱ仯�����У�Co������û�б䣬����ԭʼ��������Ϊ100g����n��Co��=![]() mol��m��Co��=59��
mol��m��Co��=59��![]() g����1000��ʱ�������������ٱ仯��˵��Co��OH��2��ȫ�ֽ⣬n��Co����n��O��=
g����1000��ʱ�������������ٱ仯��˵��Co��OH��2��ȫ�ֽ⣬n��Co����n��O��=![]() ��[��80.65-59��
��[��80.65-59��![]() ����16]=1��1��ʣ�����ɷ�CoO����500�棬n��Co����n��O��=
����16]=1��1��ʣ�����ɷ�CoO����500�棬n��Co����n��O��=��[��86.38-59��
![]() ����16]=3��4����ѧʽΪCo3O4����290�棬n��Co����n��O��=
����16]=3��4����ѧʽΪCo3O4����290�棬n��Co����n��O��=![]() ��[��89.25-59��
��[��89.25-59��![]() ����16]=2��3����ѧʽΪCo2O3��290��ʱ��Co(OH)2��ȫ��ˮ��ΪCo2O3����Ӧ�Ļ�ѧ����ʽΪ4Co(OH)2+O2
����16]=2��3����ѧʽΪCo2O3��290��ʱ��Co(OH)2��ȫ��ˮ��ΪCo2O3����Ӧ�Ļ�ѧ����ʽΪ4Co(OH)2+O2![]() 2Co2O3+4H2O ��500��ʱ����Ҫ����Ϊ��Co3O4����1000��ʱ�ķֽ����Ϊ��CoO��1molCoO��2.2mol Na2O�ڳ�벷�չ��й��ȣ��������ʺ�ɫ�ľ��壬������Ϊ�������νṹ����������Ϊ��CoO34-���侧��Ļ�ѧʽΪ��Na4CoO3���ʴ�Ϊ��4Co(OH)2+O2
2Co2O3+4H2O ��500��ʱ����Ҫ����Ϊ��Co3O4����1000��ʱ�ķֽ����Ϊ��CoO��1molCoO��2.2mol Na2O�ڳ�벷�չ��й��ȣ��������ʺ�ɫ�ľ��壬������Ϊ�������νṹ����������Ϊ��CoO34-���侧��Ļ�ѧʽΪ��Na4CoO3���ʴ�Ϊ��4Co(OH)2+O2![]() 2Co2O3+4H2O��
2Co2O3+4H2O��
Co3O4��Na4CoO3��
��5���������������õ��ġ���ҺX��������Ҫ�������ǣ�Na2SO4���ʴ�Ϊ��Na2SO4��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���в���ȷ����
A.��״���£�22.4L��������������ԭ����ԼΪ6.02��1023
B.��״���£�aL�Ķ�����̼�͵����Ļ���ﺬ�еķ�����ԼΪ![]() ��6.02��1023
��6.02��1023
C.22 g������̼���״����11.2 L �Ȼ������庬�еķ�������ͬ
D.��״���£�2.24L CCl4�к��е�ԭ����ԼΪ0.5��6.02��1023
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£����и���������ָ����Һ���ܴ����������(����)
A.��ʹ��̪������Һ��Na+��Al3+��SO42-��NO3-
B.![]() =10��12����Һ��NH4+��Na+��SO42-��Cl-
=10��12����Һ��NH4+��Na+��SO42-��Cl-
C.0.1 mol��L-1Na2SO3��Һ��NH4+��K+��ClO-��Cl-
D.0.1 mol��L-1 FeCl3��Һ��Mg2+��Al3+��MnO4-��SCN-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����������Ϣʹ(��������������)Ϊԭ�ϣ����ಽ��Ӧ�Ƹ��ص��������£�
AcHNOH����Ϣʹ![]() ������͡
������͡![]() ��
��![]() ����
����

���������͡�ĺϳ�
�ٽ�����1.0 g����Ϣʹ��ҩƬ���飬ת����˫����ƿ�С��õιܼ���8 mL 1 mol/LNaOH��95%�Ҵ���Һ����������ˮ�������ƿ������ԡ�������裬��е���������15 min��
�ڽ���ƿ�Ƴ���ԡ����ͼ��ʾ����ע����ȡ1.0 mL�ĵ����飬��μ�������Һ�С���������ƿ������ԡ����15 min��
�۽���ƿ����ԡ��̧��ȡ�������ܳ�����ɰо©�����ˣ���ȥ���ܵĵ���(ҩƬ�������)��������Һ���ã��õ�����������һ��ɰо©�����˵õ�������͡���塣
������صĺϳ�
�ٽ�������͡����ת�Ƶ�Բ����ƿ�У�����5 mL 6 mol/L���ᡣ���Ȼ���15 min��
�ڼ���NaHCO3����pHΪ6.0��6.5������ӦҺ�м�1.37 g���غ�2�����ᡣ���Ȼ���60 min��
���Ƴ���ƿ����ȴ���г������������ˣ��ñ�ˮϴ�ӣ��õ����ء�
(1)��ҩƬ�������õ���������Ϊ________________��
(2)ʵ��������ԡ���ȵ��ŵ���______________________��
(3)ʹ��ɰо©�����˵��ŵ���_________________�ҵõ��ij����ϸ��
(4)�����ܵ���ȴˮ��________(����a������b��)�˽���
(5)�������������Һ�м���NaHCO3�кͣ�Ϊ�������CO2�ݳ�������NaHCO3ʱӦ__________________��
(6)�ñ�ˮϴ�Ӹ��ع���IJ�����_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��Ӧ���������ʱ仯��ͬʱ�������������ı仯����ѧ��Ӧ�ķ�Ӧ��ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
��1����25�桢101KPa�£�1gCH3OH��������ַ�Ӧ�����ɶ�����̼�����ˮʱ�ͷų�22.68kJ�����������ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��_____��
��2����֪��֪�����ǵ�ȼ������2804kJ/mol,������������1gҺ̬ˮʱ�ų���������__________ kJ�����������λС����
��3����֪�Ȼ�ѧ����ʽC3H8(g)+5O2(g)=3CO2(g)+4H2O(l) ��H=-2220 kJ/mol��2H2(g)+O2(g)=2H2O(l) ��H=-571.6 kJ/mol
������H2��C3H8�Ļ�����干5mol,��ȫȼ��ʱ����3472.9kJ������������H2��C3H8�������Ϊ__________��
��1mol H2��2 mol C3H8��ɵĻ�����干��ȫȼ��ʱ����_________ kJ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ4A(s)+3B(g)![]() 2C(g)+ D(g)����2minB��Ũ�ȼ���0.6mol/L���Դ˷�Ӧ���ʵı�ʾ��ȷ����
2C(g)+ D(g)����2minB��Ũ�ȼ���0.6mol/L���Դ˷�Ӧ���ʵı�ʾ��ȷ����
A. ��A��ʾ�ķ�Ӧ������0.4 mol/(L��min)
B. �ֱ���B��C��D��ʾ�ķ�Ӧ�������ֵ��3��2��1
C. ��2minĩ�ķ�Ӧ���ʣ���B��ʾ��0.3mol/(L��min)
D. ����2min����B��C��ʾ�ķ�Ӧ���ʵ�ֵ������С��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����Ȼ�ѧ����ʽ��Zn(s) +![]() O2(g) ��ZnO(s) ��H1����351.1kJ/mol
O2(g) ��ZnO(s) ��H1����351.1kJ/mol
Hg(l) +![]() O2(g) �� HgO(s) ��H2����90.7kJ/mol
O2(g) �� HgO(s) ��H2����90.7kJ/mol
�ɴ˿�֪Zn(s) + HgO(s) �� ZnO(s) + Hg(l)����H3��������H3��ֵ��
A.��260.4 kJ/molB.��254.6 kJ/molC.��438.9 kJ/molD.��441.8 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С��ͬѧ��̽��ij�����ڷ���һ��ʱ�����Ϊ������������ͥ�վ�Ʒ�������Ļ��ʵ���Ҫ�ɷּ�������ʡ����ȶԸû��ʵijɷֽ��������¼��裺
a��ֻ����FeSO4
b������FeSO4��Fe2(SO4)3
c��ֻ����Fe2(SO4)3
�����ʹ����ĩ����ˮ�еõ���Һ(��ΪX)����������ʵ�飺
ʵ����� | ���� | ���� |
�� | ȡ2 mL��ҺX������1 mL 1 mol��L��1 NaOH��Һ | �������ɫ���� |
�� | ȡ2 mL��ҺX������1��KSCN | ��Һ�Ժ�ɫ |
��1���������ֱ�����������b��������__________________________��
��2����ʵ�颡��Ԥ�������Dz�����ɫ��������Ϊ����ɫ�������ֺ��ɫ������Ԥ�ڲ����������������(�û�ѧ����ʽ�����ӷ���ʽ����)_____��_____��
��3����ʵ�颢�ó��Ľ�����____________�����ʵ�颡�������Ʋ�ʵ�颡ʵ��������Ԥ��������ԭ�������_____________________________��Ϊ��һ����֤���裬С��ͬѧ����������ʵ�飺
ʵ����� | ���� | ���� |
�� | ȡ2 mL��ҺX������1��KSCN���ټ���1 mLˮ | ��Һ�Ժ�ɫ |
�� | ȡ2 mL��ҺX������1��KSCN���ټ���1 mL��ˮ | ��Һ�Ժ�ɫ����ɫ�Ȣ��� |
��4��ʵ�颤����ˮ�μӷ�Ӧ�����ӷ���ʽ��_______________________��
��5��ͨ������ʵ�飬�ɵõ��Ľ�����_____________________________������������ý�������εó���_______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com