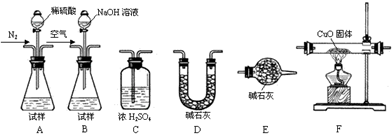
��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�����ͼI�е�һЩװ�������м��飬ʹ��������Ʒ��NaOH��Һ��Ӧ��
AlN+NaOH+H
2O�TNaAlO
2+NH
3��
���ݷ�Ӧ�������ɰ�����������ⶨ��Ʒ�еĵ�����������������������ʵ��������ȷ�����ʵijɷ֣�ʵ���е���������Բ��ƣ�
��1��ʵ���йز���Ϊ��a������ƿ�з���������AIN��Ʒ��b���ӷ�Һ©������ƿ�м��������ŨNaOH�� C������װ�õ������ԣ�d���ⶨ�ռ���ˮ�������
��ȷ�IJ���˳��Ϊ��______��
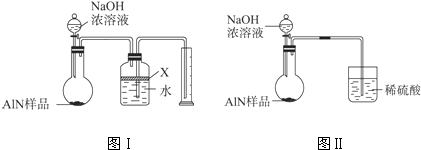
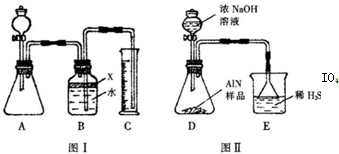
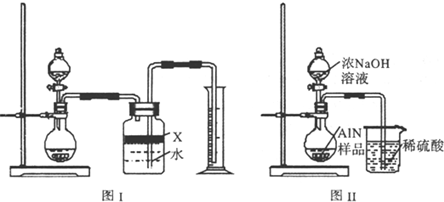
��2���������У�ͼI�����װ�������Եķ����ǣ�______��
��3�����ƿ�е��Լ�X��ѡ��______������ѡ��ı�ţ�
A������ B���ƾ� C��ֲ���� D��CCl
4��4��ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������______��
��5����ʵ���в����Ʒ������Ϊw g�����������ΪaL������£�������Ʒ��AIN����������Ϊ��______��
��6�����˸���ͼIIװ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��AIN����������������Ϊ�Ƿ���У�______ �����롰���С����������С�����ԭ����______��




 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�