
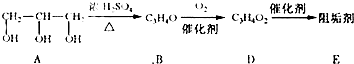
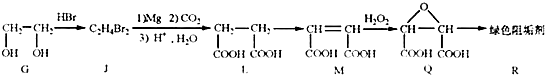
| ���� |
| �� |
| �� |
| �� |
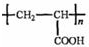
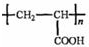
| �� |
| �� |
| �� |
| �� |
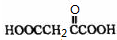

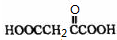


 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
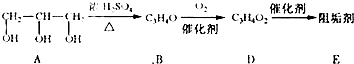
| �� |
| �� |


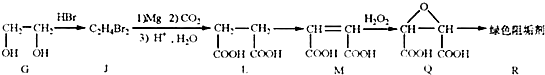
| ���� |
| �� |
| �� |
| �� |
| �� |
| �� |
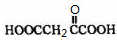 ��
��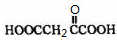 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�蹸���ɷ�ֹ��ҵ��ˮ��������������ṹ���������з�Ӧ·�߿ɵ�֪��E��R�����蹸�������ַ�Ӧ������ȥ��
��1���蹸��E���Ʊ�
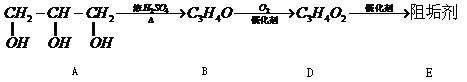
��A����������Ҫ��Ӫ������ ˮ���Ƶã�����ࡱ������֬�������ʡ���
��B�����Ƶ�![]() ��Ӧ����D���仯ѧ����ʽΪ ��
��Ӧ����D���仯ѧ����ʽΪ ��
��D���Ӿ۷�Ӧ����E��E�Ľṹ��ʽΪ ��
��2���蹸��R���Ʊ�
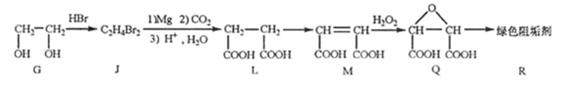
��G��JΪȡ����Ӧ��J�Ľṹ��ʽΪ ��www.k@s@5@u.com ��#��#��#Դ#��
����L�Ʊ�M�ķ�Ӧ��������Ϊ��
![]()
�� ���û�ѧ����ʽ��ʾ����
��1mol Q��ͬ���칹��T��̼����֧����������![]() ��Һ���ò���2mol
��Һ���ò���2mol ![]() ��T�Ľṹ��ʽΪ ��ֻдһ�֣���
��T�Ľṹ��ʽΪ ��ֻдһ�֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�蹸���ɷ�ֹ��ҵ��ˮ��������������ṹ���������з�Ӧ·�߿ɵ�֪��E��R�����蹸�������ַ�Ӧ������ȥ��
��1���蹸��E���Ʊ�
��A����������Ҫ��Ӫ������ ˮ���Ƶã�����ࡱ������֬�������ʡ���
��B�����Ƶ�![]() ��Ӧ����D���仯ѧ����ʽΪ ��
��Ӧ����D���仯ѧ����ʽΪ ��
��D���Ӿ۷�Ӧ����E��E�Ľṹ��ʽΪ ��
��2���蹸��R���Ʊ�
��G��JΪȡ����Ӧ��J�Ľṹ��ʽΪ ��www..com
����L�Ʊ�M�ķ�Ӧ��������Ϊ��
![]()
�� ���û�ѧ����ʽ��ʾ����
��1mol Q��ͬ���칹��T��̼����֧����������![]() ��Һ���ò���2mol
��Һ���ò���2mol 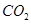 ��T�Ľṹ��ʽΪ ��ֻдһ�֣���
��T�Ľṹ��ʽΪ ��ֻдһ�֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��߿���ѧ�������ר��ʮ�� �л������� ���ͣ������
��16�֣��蹸���ɷ�ֹ��ҵ��ˮ��������������Ṹ���������з�Ӧ·�߿ɵõ�E��R�����蹸�������ַ�Ӧ������ȥ����
��1���蹸��E���Ʊ�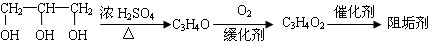
�� A����������Ҫ��Ӫ������________ˮ���Ƶã�����ࡱ������֬�������ʡ�����
�� B�����Ƶ�Cu(OH)2��Ӧ����D���仯ѧ����ʽΪ__________ ____��
____��
�� D���Ӿ۷�Ӧ����E��E�Ľṹ��ʽΪ______________��[��Դ:ѧ����ZXXK]
��2���蹸��R���Ʊ�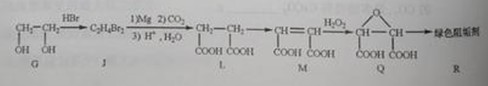
�� Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��
Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��
��Jת��ΪL�Ĺ����У�L���������ӵ�̼ԭ����Դ�� __________________��
����L�Ʊ�M�ķ�Ӧ��������Ϊ��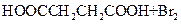

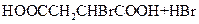 ��_____��_____���û�ѧ����ʽ��ʾ����
��_____��_____���û�ѧ����ʽ��ʾ����
��1 mol Q��ͬ���칹��T��̼����֧����������NaHCO3��Һ���ò���2 mol CO2��T�Ľṹ��ʽΪ__________��ֻдһ�֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010����ͨ�ߵ�ѧУ����ȫ��ͳһ�������ۻ�ѧ���֣�������� ���ͣ������
��16�֣��蹸���ɷ�ֹ��ҵ��ˮ��������������Ṹ���������з�Ӧ·�߿ɵõ�E��R�����蹸�������ַ�Ӧ������ȥ����
��1���蹸��E���Ʊ�
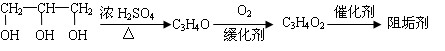
�� A����������Ҫ��Ӫ������________ˮ���Ƶã�����ࡱ������֬�������ʡ�����
�� B�����Ƶ�Cu(OH)2��Ӧ����D���仯ѧ����ʽΪ______________��
�� D���Ӿ۷�Ӧ����E��E�Ľṹ��ʽΪ______________��[��Դ:ZXXK]
��2���蹸��R���Ʊ�
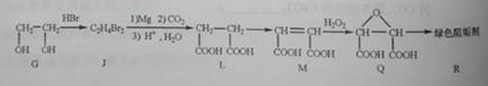
��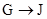 Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��
Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��
��Jת��ΪL�Ĺ����У�L���������ӵ�̼ԭ����Դ�� __________________��
����L�Ʊ�M�ķ�Ӧ��������Ϊ��
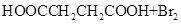
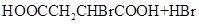 ��_____��_____���û�ѧ����ʽ��ʾ����
��_____��_____���û�ѧ����ʽ��ʾ����
��1 mol Q��ͬ���칹��T��̼����֧����������NaHCO3��Һ���ò���2 mol CO2��T�Ľṹ��ʽΪ__________��ֻдһ�֣���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com