

| ||
| ||
| ||
| ||
| 10-5.1ЎБ10-5.1 |
| 0.1 |
| ||
| ||
| 10-5.1ЎБ10-5.1 |
| 0.1 |
| ||
| ||



| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
| јУИИ |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2013-2014С§Дкєю±±КЎёЯИэЙПС§ЖЪЖЪД©їјКФАнЧЫ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєМоїХМв
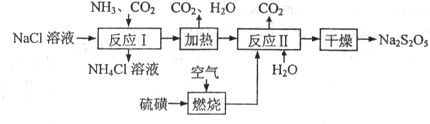
Ѕ№СЗБтЛбДЖЈЁNa2S2O5Ј©іЈУГЧчКіЖ·ЖЇ°ЧјБЎЈЖдЦЖ±ё№¤ТХБчіМИзПВЈє
ТСЦЄЈє·ґУ¦ўт°ьє¬2NaHSO3 Na2S2O5Ј«H2OµИ¶аІЅ·ґУ¦ЎЈ
Na2S2O5Ј«H2OµИ¶аІЅ·ґУ¦ЎЈ
ЈЁ1Ј©КµСйКТЦЖИЎ°±ЖшµД»ЇС§·ЅіМКЅЈє ЎЈ
ЈЁ2Ј©Ў°ЧЖЙХЎ±К±·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅЈє ЎЈ
ЈЁ3Ј©ТСЦЄNa2S2O5УлПЎБтЛб·ґУ¦·ЕіцSO2Ј¬ЖдАлЧУ·ЅіМКЅОЄЈє ЎЈ
ЈЁ4Ј©ё±ІъЖ·XµД»ЇС§КЅКЗЈє Ј»їЙС»·АыУГµДОпЦККЗЈє_________єН_______ЎЈ
ЈЁ5Ј©ОЄБЛјхЙЩІъЖ·Na2S2O5ЦРФУЦКє¬БїЈ¬РиїШЦЖ·ґУ¦ўтЦРЖшМеУл№ММеµДОпЦКµДБїЦ®±ИФјОЄ ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2013-2014С§ДкЅЛХКЎёЯИэЙПС§ЖЪ12ФВФВїј»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєМоїХМв
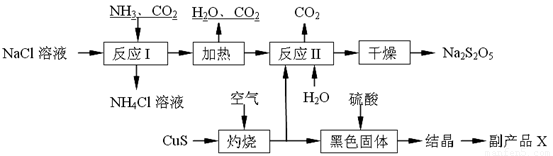
Ѕ№СЗБтЛбДЖЈЁNa2S2O5Ј©іЈУГЧчКіЖ·ЖЇ°ЧјБЎЈЖдЦЖ±ё№¤ТХБчіМИзПВЈє

ТСЦЄЈє·ґУ¦ўт°ьє¬2NaHSO3 Na2S2O5Ј«H2OµИ¶аІЅ·ґУ¦ЎЈ
Na2S2O5Ј«H2OµИ¶аІЅ·ґУ¦ЎЈ
ЈЁ1Ј©КµСйКТЦЖИЎ°±ЖшµД»ЇС§·ЅіМКЅЈє ЎЈ
ЈЁ2Ј©·ґУ¦IµД»ЇС§·ЅіМКЅОЄЈє ЎЈ
ЈЁ3Ј©Ў°ЧЖЙХЎ±К±·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅЈє ЎЈ
ЈЁ4Ј©ТСЦЄNa2S2O5УлПЎБтЛб·ґУ¦·ЕіцSO2Ј¬ЖдАлЧУ·ЅіМКЅОЄЈє ЎЈ
ЈЁ5Ј©ё±ІъЖ·XµД»ЇС§КЅКЗ ЎЈ
ЈЁ6Ј©ОЄБЛјхЙЩІъЖ·Na2S2O5ЦРФУЦКє¬БїЈ¬РиїШЦЖ·ґУ¦ўтЦРЖшМеУл№ММеµДОпЦКµДБїЦ®±ИФјОЄ ЎЈјмСйІъЖ·ЦРє¬УРМјЛбДЖФУЦКЛщРиКФјБКЗ ЈЁМо±аєЕЈ©
ўЩЛбРФёЯГМЛбјШ ўЪЖ·ємИЬТє ўЫіОЗеКЇ»ТЛ®
ўЬ±ҐєНМјЛбЗвДЖИЬТє ўЭNaOH ўЮПЎБтЛб
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2013-2014С§Дк№г¶«КЎёЯИэµЪИэґОФВїјАнЧЫ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєМоїХМв
Ѕ№СЗБтЛбДЖЈЁNa2S2O5Ј©іЈУГЧчКіЖ·ЖЇ°ЧјБЎЈЖдЦЖ±ё№¤ТХБчіМИзПВЈє
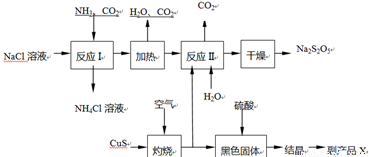
ТСЦЄЈє·ґУ¦ўт°ьє¬2NaHSO3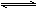 Na2S2O5Ј«H2OµИ¶аІЅ·ґУ¦ЎЈ
Na2S2O5Ј«H2OµИ¶аІЅ·ґУ¦ЎЈ
ЈЁ1Ј©КµСйКТЦЖИЎ°±ЖшµД»ЇС§·ЅіМКЅЈє ЎЈ
ЈЁ2Ј©Ў°ЧЖЙХЎ±К±·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅЈє ЎЈ
ЈЁ3Ј©ТСЦЄNa2S2O5УлПЎБтЛб·ґУ¦·ЕіцSO2Ј¬ЖдАлЧУ·ЅіМКЅОЄЈє ЎЈ
ЈЁ4Ј©ё±ІъЖ·XµД»ЇС§КЅКЗЈє Ј»їЙС»·АыУГµДОпЦККЗЈє_____________ЎЈ
ЈЁ5Ј©ОЄБЛјхЙЩІъЖ·Na2S2O5ЦРФУЦКє¬БїЈ¬РиїШЦЖ·ґУ¦ўтЦРЖшМеУл№ММеµДОпЦКµДБїЦ®±ИФј
ОЄ ЎЈ
ЈЁ6Ј©јмСйІъЖ·ЦРє¬УРМјЛбДЖФУЦКЛщРиКФјБКЗ ЈЁМо±аєЕЈ©
ўЩЛбРФёЯГМЛбјШ ўЪЖ·ємИЬТє ўЫіОЗеКЇ»ТЛ® ўЬ±ҐєНМјЛбЗвДЖИЬТє ўЭNaOH ўЮПЎБтЛб
Ійїґґр°ёєНЅвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com