
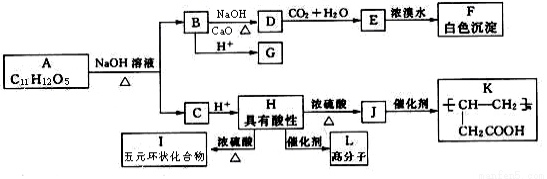
��ѡ��5���л���ѧ������ij�л���A��ˮ��Һ�����ԣ���FeCl3��Һ����ɫ��A���ӽṹ�в��������������������ϵ�һ�ȴ���ֻ������, A�������л����������ͼ��ʾ��ת����ϵ��
��֪�� 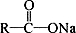 + NaOH
+ NaOH  R��H + Na2CO3
R��H + Na2CO3
�Իش��������⣺
��1��B��ѧʽ________________��G�Ļ�ѧ������___________________��
��2��H��I��Ӧ����Ϊ______________��J���������ŵ�����Ϊ___________��
��3��д��H��L ��Ӧ�Ļ�ѧ����ʽ__________________________________��
��4��A�Ľṹ��ʽ_________________________��
��5��F��ͬ���칹���к��б����ҹ�������ͬ�����ʹ���__________�֣�������F�������к˴Ź��������������壬�ҷ������Ϊ1�U2����___________��д�ṹ��ʽ����
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ����ϳ���ʯ�ŵ�һ��ѧ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪X2��Y2��Z2��W2�������ʵ���������Ϊ��W2>Z2>X2>Y2������������ԭ��Ӧ�ܷ������ǣ� ��
A��2W- + Z2 = 2Z- +W2 B��2X- + Z2 = 2Z- + X2
C��2Y- + Z2 = 2Z- + Y2 D��2Z- + X2 = 2X- + Z2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ����ϳ���ʯ�ŵ�һ��ѧ�߶�����������ѧ���������棩 ���ͣ�ѡ����
����˵����ȷ����
A�������£���pH��3�Ĵ�����Һϡ�͵�ԭ�����10������Һ��pH��4
B����ͬ�¶��£��������Ȼ�������ֱ���룺�� ����ˮ�� �� 0.1 mol��L��1�Ȼ�þ��Һ���� 0.1 mol��L��1���ᡢ�� 0.1 mol��L��1��������Һ�У�����ܽ��Ag��Ũ�ȣ��٣� �ۣ��ܣ���
C�������£�pH��3��CH3COOH��Һ��pH��11��NaOH��Һ�������Ϻ���Һ��pH��7
D��Ϊȷ��ij��H2A��ǿ�ỹ�����ᣬ�ɲⶨ����ʱNaHA��Һ��pH����pH>7����H2A�������pH<7����H2A��ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016������ʡ��У������ѧ��12���¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��NA��ʾ�����ӵ�����������˵����ȷ����( )
��18g D2O���еĵ�����Ϊ10NA
��ͬ�¡�ͬѹ�£���ͬ����ķ��������������ԭ�������
�۱�״���£�11.2L�����������ϵĵ���������������ԭ����ΪNA
���ڱ�״���£�22.4LSO3�����ʵ���Ϊ1mol
��4��ʱ5.4mL��ˮ������ԭ������Ϊ0.9NA
��60gSiO2�������2 NA��Si��O��
��1mol Na2O2��ˮ��ȫ��Ӧʱת�Ƶ�����Ϊ2NA
��200mL 1mol/L Al2(SO4)3��Һ�У�Al3����SO42����������ΪNA
A���ۢݢޢ� B���٢ڢܢ� C���ۢ� D���ۢܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����������·��һ��ѧ��һ�϶ο���ѧ�Ծ��������棩 ���ͣ��ƶ���
A��B��C��D��F�������ʵ���ɫ��Ӧ��Ϊ��ɫ��A��B��C��D�����ᷴӦ������E������B������һ�ֿ�ȼ���壬��C��D������һ����ɫ��ζ����H����������ʹ����ʯ��ˮ����ǡ�D��A�ɷ�Ӧ����C��F��HҲ�ɷ�Ӧ����C����һ����ɫ��ζ���塣��ش��������⣺
��1��д��A��B��E�Ļ�ѧʽ��A_______________��B_______________��E_______________
��2��д��F��H��Ӧ�Ļ�ѧ����ʽ ��
��3����Ҫ��д����ʽ��
�� ���ȹ���D������Ӧ�Ļ�ѧ��Ӧ����ʽ ��
�� C���������ᷴӦ�����ӷ���ʽ ��
�� A��Һ���������Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����������·��һ��ѧ�߶��϶ο�����ѧ���������棩 ���ͣ������
I�����㣨���¾�Ϊ�����£���1��pH��2��H2SO4��Һ��ˮ�������c(H��)��__________
��2��pH��2��NH4Cl��Һ��ˮ�������c(H��)��__________
��3�� pH��2��H2SO4��Һ��pH��5 H2SO4�������1��9��ϣ���Ϻ���Һ��pH��_________
��4�� pH��12��NaOH��Һ��pH��4�� H2SO4��Ϻ���Һ�����ԣ���V(NaOH)��V(H2SO4)= _________
II��25 ��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | 1.7��10��5 | K1��4.3��10��7 K2��5.6��10��1 1 | 3.0��10��8 |
��ش��������⣺
��1��CH3COOH��H2CO3��HClO��������ǿ������˳��Ϊ__________________
��2��ͬŨ�ȵ�CH3COO����HCO 3-��CO32-��ClO�����H����������ǿ������˳��Ϊ___________
3-��CO32-��ClO�����H����������ǿ������˳��Ϊ___________
��3�����Ϊ10 mL pH��2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1 000 mL��ϡ������pH�仯��ͼ��ʾ����HX�ĵ���ƽ�ⳣ��______(����ڡ��������ڡ���С�ڡ�)����ĵ���ƽ�ⳣ����������__________________________________________��
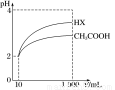
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016���㽭ʡ���ˡ����ݡ�������У�����ϵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ����
A����2H2(g)+O2(g) =2H2O(g)��H=-483.6 kJ��mol��1����H2�ı�ȼ����Ϊ-241.8 kJ��mol��1
B����C(ʯī��s) =C(���ʯ��s) ��H>0����ʯī�Ƚ��ʯ�ȶ�
C����֪NaOH(aq)��HCl(aq)=NaCl(aq)��H2O(l) ��H��-57.4 kJ��mol-1����20.0g NaOH������ϡ������ȫ�кͣ��ų�28.7 kJ������
D����֪2C(s)��2O2(g) =2CO2(g) ��H1��2C(s)��O2(g) =2CO(g) ��H2����H1>��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫���ڸ���ѧ��һ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
�Ȼ������ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ����γ���NaCl�⣬����������MgCl2��CaCl2��Na2SO4�Լ���ɳ�����ʡ������Ǵ����ᴿ�IJ������̡�

��ѡ�Լ���Na2CO3��Һ��K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ������NaCl��Һ��
��1������ȥ��ҺI�е�MgCl2��CaCl2��Na2SO4�����ṩ���Լ���ѡ��a���������Լ������μ�˳������Ϊ��������NaOH��Һ��_________��________���� �ɵij������еijɷ�Ϊ��ɳ��________��
�ɵij������еijɷ�Ϊ��ɳ��________��
��2������Һ�м����ᷢ����Ӧ�����ӷ���ʽ��____________________________________��
��3������ҺII�����õ�����ʱ��������ʹ�õ��IJ���������_________�֡�
��4���õ���NaCl��Ʒ���ⶨ�����京�е�NaCl�������NaCl������һ�£�ijͬѧ��Ϊ�˴�ʵ��dz��ɹ�û�������Ƿ�ͬ�����Ĺ۵㣿_______����ǡ������������������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016���㽭�ٺ�̨����ѧ������ѧ�ڵ�����ͳ����ѧ�Ծ��������棩 ���ͣ�ѡ����
��NA��������٤����������ֵ��������˵����ȷ����
A�����³�ѹ�£�1mol�������еĺ��������Ϊ4NA
B��0.25mol Na2O2�к��е���������Ϊ0.5NA
C��50ml 18.4mol/LŨ����������ͭ�ȣ���������0.46NA
D��25��ʱ��7g C2H4��C3H6�Ļ�������У�����NA��C��H��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com