
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
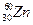 ��
�� ��ԭ�Ӿ��˾ۺϷų�һ����Ŀ�����ӣ��õ�112�ŵ���Ԫ�أ���Ԫ��ԭ�ӵ�������Ϊ267�������йظ�Ԫ�ص�˵����ȷ����
��ԭ�Ӿ��˾ۺϷų�һ����Ŀ�����ӣ��õ�112�ŵ���Ԫ�أ���Ԫ��ԭ�ӵ�������Ϊ267�������йظ�Ԫ�ص�˵����ȷ����| A��λ�ڵ������ڵڢ�A�� |
| B����ԭ���У���������������֮��Ϊ43 |
| C��ԭ�Ӻ����6�����Ӳ� |
| D�����С����ԡ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH2= CH2 | B��N2 | C��Cl2 | D��C2H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���ڢۢ� | C���٢ڢ� | D���ܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
|
���ж�һЩ��ʵ�Ľ��ʹ������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
|
| A���٢ۢ� | B���٢ۢ� | C���ۢܢ� | D���ڢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
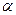 -����ɢ���������˶��ķ��ֶ���ԭ�ӽṹģ�͵Ľ��������˹���
-����ɢ���������˶��ķ��ֶ���ԭ�ӽṹģ�͵Ľ��������˹����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com