
����Ŀ����֪A��B��C��D��ԭ������������������ֶ���������Ԫ�أ�A������������������������Bԭ�ӵļ۵����Ų�Ϊnsnnpn��D�ǵؿ��к�������Ԫ�ء�E�ǵ�������p����Ԫ���������ֻ��2�ԳɶԵ��ӣ�F��29��Ԫ�ء�
��1��B��C��D��Ԫ�ص�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ű�ʾ��
��2��BD32-����ԭ���ӻ����������Ϊ________�ӻ���CA4+�Ŀռ乹��Ϊ______________��
��3����̬Eԭ�ӵļ۵����Ų�ͼ______________________________��
��4��1mol BC���к��Цм�����ĿΪ______________��
��5���Ƚ�D��EԪ������⻯��ķе�ߵͣ� ���û�ѧʽ��ʾ����
��6��C��F��Ԫ���γɵ�ij������ľ����ṹ��ͼ��ʾ������ΪCԭ�ӡ���û�����Ļ�ѧʽ�� ��Cԭ�ӵ���λ���� ��������Cԭ�Ӻ�Fԭ�Ӽ�ľ���Ϊa cm������٤������ΪNA����þ�����ܶ�Ϊ________________g��cm3���ú�a��NA�ķ��ű�ʾ����

���𰸡���1��N��O��C
��2��sp2 ��������
��3��![]()
��4��2NA
��5��H2O��H2Se
��6��Cu3N 6 103/4a3NA
��������
������������������Ϣ�� A��B��C��D��ԭ������������������ֶ���������Ԫ�أ�A������������������������Bԭ�ӵļ۵����Ų�Ϊnsnnpn��D�ǵؿ��к�������Ԫ�أ���AΪH��BΪ̼��DΪ������CΪ��Ԫ�أ�E�ǵ������ڵ�p��Ԫ���������ֻ��2�ԳɶԵ��ӣ�EΪSe��FԪ�ص�ԭ������Ϊ29��FΪͭ����
��1��ͬ�����������ҵ�һ��������С����NԪ�ص�2p������Ӵ��ڰ����״̬���ȶ���ǿ����һ�����ܴ�����Ԫ�أ���B��C��D��Ԫ�ص�һ�������ɴ�С��˳��ΪN��O��C��
��2��CO32-����ԭ��̼ԭ�ӵļ۲���Ӷ�����3+![]() ��������ӻ����������Ϊsp2�ӻ���NH4+�Ŀռ乹��Ϊ�������塣
��������ӻ����������Ϊsp2�ӻ���NH4+�Ŀռ乹��Ϊ�������塣
��3��Se��ԭ��������34������ݺ�������Ų����ɿ�֪��̬Seԭ�ӵļ۵����Ų�ͼΪ![]() ��
��
��4��CN-������C��N��ΪC��N��1mol CN���к��Цм�����ĿΪ2NA��
��5��ˮ���Ӽ������������⻯��е�ΪH2O��H2Se��
��6�����ݾ����Ľṹ��֪������Cu���������![]() ��������N�������Ϊ
��������N�������Ϊ![]() ������������Ļ�ѧʽCu3N�������N3-��1/8����Ӧ������������3��Cu+��1/4������N3-��Cu+=1/8��3/4=1��6������λ����6�������������V=��2a��3cm3������Ϊ206/NA�����ܶ�Ϊ103/4a3NA��
������������Ļ�ѧʽCu3N�������N3-��1/8����Ӧ������������3��Cu+��1/4������N3-��Cu+=1/8��3/4=1��6������λ����6�������������V=��2a��3cm3������Ϊ206/NA�����ܶ�Ϊ103/4a3NA��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������½ṹ��ԭ�ӣ�һ����������Ԫ�ص��ǣ� ��
A. �������3��δ�ɶԵ��ӵ�ԭ��
B. ���������Ų�Ϊns2��ԭ��
C. �������δ�ɶԵ��ӵ�ԭ��
D. �������8�����ӵ�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ���������ȷ�Ӧ������ ��
A��þ�����ᷴӦ�ų�����
B����������������ķ�Ӧ
C�����ڿ�����������ȼ��
D��Ba(OH��28H2O��NH4Cl��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л�������һ����Ҫ�����ϣ������ɵ���A�� CH2=C��CH3��COOCH3 ��ͨ���Ӿ۷�Ӧ�Ƶã���֪����A�ɷ�����ͼ��ʾ��ת����ϵ��

��֪������������ϡH2SO4�з���ˮ�⣺
CH3C18OOCH2CH3 + H2O ![]() CH3C18OOH + CH3CH2OH
CH3C18OOH + CH3CH2OH
��1���л������Ľṹ��ʽ�� ��
��2��B�����к��еĹ������� ������������
��3����Bת��ΪC�ķ�Ӧ���� �����������
��������Ӧ �ڻ�ԭ��Ӧ �ۼӳɷ�Ӧ ��ȡ����Ӧ
��4��C��һ�ȴ���D�� �ֽṹ����COOH�ϵ��ⲻ���Cl2����ȡ����Ӧ����
��5����A����B�Ļ�ѧ����ʽ�� ��
��6��CH3CH2OH��CH3OH��ͬϵ���д��CH3CH2OH ��O2��ͭ�����������ȵ������·�����Ӧ�Ļ�ѧ��Ӧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л�������G�Ǻϳ�ά������ҩ����м��壬��ϳ�·�����£�

����A��F�ֱ����һ���л�������ϳ�·���в��ֲ��P��Ӧ��������ȥ��֪��

GΪ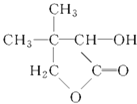 ��
��
��ش��������⣺
��1��G�ķ���ʽ_____________��D�й����ŵ�������_________��
��2����������Ӧ�Ļ�ѧ����ʽΪ__________________________��
��3����������Ӧ�Ļ�ѧ����ʽΪ__________________________��
��4��д��F�Ľṹ��ʽ_____________��
��5������~������Ӧ�����ڼӳɷ�Ӧ����___________________������ȡ����Ӧ����
__________________________�����������
��6��ͬʱ��������������E��ͬ���칹����_____________�֡�
��ֻ��һ�ֹ����ţ�
����״�ṹ���ޡ�O��O����
���˴Ź�������ֻ��2��塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��������ˮ��Cr2O72-����Ⱦ�����ȼ����Լ�ʹ֮��ΪCr3+�����Լ�Ϊ
A. NaOH��Һ B. FeCl3��Һ C. ���� D. Na2SO3��H2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�

����ȡ50mL 0.25mol/L H2SO4��Һ����С�ձ��У������¶ȣ�
����ȡ50mL 0.55mol/L NaOH��Һ�������¶ȣ�
�۽�NaOH��Һ����С�ձ���,��Ͼ��Ⱥ�������Һ�¶ȡ���ش�
��1������ͼ��ʾ������A��������_________ ______��
��2��NaOH��Һ�Թ�����ԭ�� ____________________________��
��3������NaOH��Һ����ȷ������_______������ĸ����
A���ز������������� B��һ��Ѹ�ټ��� C�������μ���
��4��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������ ________ __________________��
��5������Һ���ܶȾ�Ϊ1g��cm-3,�кͺ���Һ�ı�����c=4.18 J����g���棩-1�������ʵ������д�����к��ȵ��Ȼ�ѧ����ʽ_______________________________

��6������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ��
a��ʵ��װ�ñ��¡�����Ч����
b���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
c�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��7����������ȷ����ȡ���Һ������¶ȣ�_______ ____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����в��ϵ����Լ���;˵���������(
A���ߴ�������������ά��ʵ�ֹ���ź�ת��
B��ʯӢ����ɫ���ľ������ͨ����˵��ˮ��������Ҫ�ɷ��Ƕ�������
C�����ά�����������ǿ���Ƿdz��õ�ͨѶ����
D���轺��ף�����ˮ����ǿ��������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�� �����йؼ��������ȣ�Sr��Ԫ�ص��ʼ��仯����������У���ȷ���ǣ� ��
A. ������ˮ��Ӧ������Ӧ����
B. �����������ӻ����������ˮ
C. �������ȼ�������������þ�ļ���
D. �����ȵ�������ɫ������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com