
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)һ���¶ȡ�ѹǿ�£�һʢ��NO2���Թܵ�����ʢ����ˮ��ˮ����Թ���ˮ�����ռ�Թ��ݻ���________________��
(2)��ͬ״���£�30 mL N2��NO2�Ļ������ͨ�뵹����ˮ����ʢ��ˮ����Ͳ�У�ʣ������16 mL����ԭ���������N2��NO2���������________________��
(3)NO��NO2ʢ��һ�Թܵ�����ʢ����ˮ��ˮ������������СΪԭ����3/5��������������________________��
(4)һ�������£����������NO��NO2�����������Թܣ�������ʢ����ˮ��ˮ�����У���ַ�Ӧ��ʣ����������Ϊԭ�������________________��
(5)һ�������£����������O2��NO�����������Թܣ�������ʢ����ˮ��ˮ�����ַ�Ӧ��ʣ����������Ϊԭ�������________________��
(6)һ�������£����������O2��NO2�����������Թܣ�������ʢ����ˮ��ˮ�����ַ�Ӧ��ʣ����������Ϊԭ�������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��)
���û�ѧ��Ӧԭ���о���������Ԫ�صĵ��ʼ��仯����ķ�Ӧ����Ҫ���塣
��(1)һ���¶Ⱥ�ѹǿ�£���ӦN2(g) + 3H2(g) 2NH3(g)�ﵽ��ѧƽ��״̬������ƽ����ϵ��ͨ�������ƽ�� (����������ҡ�����)�ƶ�����ʹ�û��Ը�ǿ�Ĵ������÷�Ӧ�Ħ�H (���������С�����ı䡱)��
(2)��һ���¶Ⱥ�ѹǿ�£���֪��
O2 (g) = O2+(g) + e-��![]() H1= 1175.7 kJ/mol
H1= 1175.7 kJ/mol
PtF6 (g) + e-= PtF6- (g)��![]() H2= �D771.1 kJ/mol
H2= �D771.1 kJ/mol
O2PtF6 (s)= O2+ (g) + PtF6- (g)�� H3=482.2 kJ/mol
H3=482.2 kJ/mol
��ӦO2 (g) + PtF6 (g) =O2PtF6 (s)��![]() H=_____________ kJ/mol��
H=_____________ kJ/mol��
�����г����µ�������Һ����0.01 mol/L CH3COOH��Һ����0.01 mol/L HCl��Һ����pH=12�İ�ˮ����pH=12��NaOH��Һ����0.01mol/L CH3COOH��Һ��pH=12�İ�ˮ�������Ϻ�������Һ����0.01 mol/L HCl��Һ��pH=12��NaOH��Һ��������������Һ��
(1)�ݡ�����Һ�Ƚϣ�pH�ϴ���� ��
(2)������Һ�У�ˮ�ĵ���̶���ͬ����______________��
(3)���ڡ��ۻ�Ϻ�������ҺpH=7����������Һ���������________ ��(ѡ�����������������)��
(4)ϡ����ͬ��������Һ��pH���� �ڣ��� ��(ѡ�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011������������2011�������һ�ε��в��ԣ����ۣ���ѧ�Ծ� ���ͣ������
(16��)
���û�ѧ��Ӧԭ���о���������Ԫ�صĵ��ʼ��仯����ķ�Ӧ����Ҫ���塣 ��(1)һ���¶Ⱥ�ѹǿ�£���ӦN2(g) + 3H2(g)
��(1)һ���¶Ⱥ�ѹǿ�£���ӦN2(g) + 3H2(g)  2NH3(g)�ﵽ��ѧƽ��״̬������ƽ����ϵ��ͨ�������ƽ�� (����������ҡ�����)�ƶ�����ʹ�û��Ը�ǿ�Ĵ������÷�Ӧ�Ħ�H (���������С�����ı䡱)��
2NH3(g)�ﵽ��ѧƽ��״̬������ƽ����ϵ��ͨ�������ƽ�� (����������ҡ�����)�ƶ�����ʹ�û��Ը�ǿ�Ĵ������÷�Ӧ�Ħ�H (���������С�����ı䡱)�� (2)��һ���¶Ⱥ�ѹǿ�£���֪��
(2)��һ���¶Ⱥ�ѹǿ�£���֪��
O2 (g) = O2+ (g) + e-�� H1=" 1175.7" kJ/mol
H1=" 1175.7" kJ/mol PtF6 (g) + e- = PtF6- (g)��
PtF6 (g) + e- = PtF6- (g)�� H2=" �D771.1" kJ/mol
H2=" �D771.1" kJ/mol O2PtF6 (s) = O2+ (g) + PtF6- (g)��
O2PtF6 (s) = O2+ (g) + PtF6- (g)��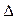 H3=" 482.2" kJ/mol
H3=" 482.2" kJ/mol ��ӦO2 (g) + PtF6 (g) = O2PtF6(s)��
��ӦO2 (g) + PtF6 (g) = O2PtF6(s)�� H="_____________" kJ/mol��
H="_____________" kJ/mol�� �����г����µ�������Һ����0.01 mol/L CH3COOH��Һ����0.01 mol/L HCl��Һ����pH=12�İ�ˮ����pH=12��NaOH��Һ����0.01 mol/L CH3COOH��Һ��pH=12�İ�ˮ�������Ϻ�������Һ����0.01 mol/L HCl��Һ��pH=12��NaOH��Һ��������������Һ��
�����г����µ�������Һ����0.01 mol/L CH3COOH��Һ����0.01 mol/L HCl��Һ����pH=12�İ�ˮ����pH=12��NaOH��Һ����0.01 mol/L CH3COOH��Һ��pH=12�İ�ˮ�������Ϻ�������Һ����0.01 mol/L HCl��Һ��pH=12��NaOH��Һ��������������Һ��
(1)�ݡ�����Һ�Ƚϣ�pH�ϴ���� ��
(2)������Һ�У�ˮ�ĵ���̶���ͬ����______________��
(3)���ڡ��ۻ�Ϻ�������ҺpH=7����������Һ���������________ ��(ѡ�����������������)��
(4)ϡ����ͬ��������Һ��pH���� �ڣ��� ��(ѡ�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ������������2010-2011ѧ�������һ�ε��в��ԣ����ۣ���ѧ�Ծ� ���ͣ������
(16��)
���û�ѧ��Ӧԭ���о���������Ԫ�صĵ��ʼ��仯����ķ�Ӧ����Ҫ���塣
��(1)һ���¶Ⱥ�ѹǿ�£���ӦN2(g) + 3H2(g)  2NH3(g)�ﵽ��ѧƽ��״̬������ƽ����ϵ��ͨ�������ƽ��
(����������ҡ�����)�ƶ�����ʹ�û��Ը�ǿ�Ĵ������÷�Ӧ�Ħ�H
(���������С�����ı䡱)��
2NH3(g)�ﵽ��ѧƽ��״̬������ƽ����ϵ��ͨ�������ƽ��
(����������ҡ�����)�ƶ�����ʹ�û��Ը�ǿ�Ĵ������÷�Ӧ�Ħ�H
(���������С�����ı䡱)��
 (2)��һ���¶Ⱥ�ѹǿ�£���֪��
(2)��һ���¶Ⱥ�ѹǿ�£���֪��
O2 (g) = O2+
(g) + e-��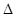 H1= 1175.7 kJ/mol
H1= 1175.7 kJ/mol
PtF6 (g) + e-
= PtF6- (g)��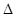 H2= �D771.1 kJ/mol
H2= �D771.1 kJ/mol
 O2PtF6 (s)
= O2+ (g) + PtF6- (g)��
O2PtF6 (s)
= O2+ (g) + PtF6- (g)�� H3=
482.2 kJ/mol
H3=
482.2 kJ/mol
��ӦO2 (g) + PtF6 (g) =
O2PtF6 (s)��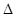 H=_____________ kJ/mol��
H=_____________ kJ/mol��
 �����г����µ�������Һ����0.01 mol/L CH3COOH��Һ����0.01 mol/L HCl��Һ����pH=12�İ�ˮ����pH=12��NaOH��Һ����0.01
mol/L CH3COOH��Һ��pH=12�İ�ˮ�������Ϻ�������Һ����0.01 mol/L HCl��Һ��pH=12��NaOH��Һ��������������Һ��
�����г����µ�������Һ����0.01 mol/L CH3COOH��Һ����0.01 mol/L HCl��Һ����pH=12�İ�ˮ����pH=12��NaOH��Һ����0.01
mol/L CH3COOH��Һ��pH=12�İ�ˮ�������Ϻ�������Һ����0.01 mol/L HCl��Һ��pH=12��NaOH��Һ��������������Һ��
(1)�ݡ�����Һ�Ƚϣ�pH�ϴ���� ��
(2)������Һ�У�ˮ�ĵ���̶���ͬ����______________��
(3)���ڡ��ۻ�Ϻ�������ҺpH=7����������Һ���������________ ��(ѡ�����������������)��
(4)ϡ����ͬ��������Һ��pH���� �ڣ��� ��(ѡ�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���û�ѧ��Ӧԭ���о���������Ԫ�صĵ��ʼ��仯����ķ�Ӧ����Ҫ���塣
��(1)һ���¶Ⱥ�ѹǿ�£���ӦN2(g) + 3H2(g) ![]() 2NH3(g)�ﵽ��ѧƽ��״̬������ƽ����ϵ��ͨ�������ƽ�� (����������ҡ�����)�ƶ�����ʹ�û��Ը�ǿ�Ĵ������÷�Ӧ�Ħ�H (���������С�����ı䡱)��
2NH3(g)�ﵽ��ѧƽ��״̬������ƽ����ϵ��ͨ�������ƽ�� (����������ҡ�����)�ƶ�����ʹ�û��Ը�ǿ�Ĵ������÷�Ӧ�Ħ�H (���������С�����ı䡱)��
(2)��һ���¶Ⱥ�ѹǿ�£���֪��
O2 (g) = O2+ (g) + e-��![]() H1= 1175.7 kJ/mol
H1= 1175.7 kJ/mol
PtF6 (g) + e- = PtF6- (g)��![]() H2= �D771.1 kJ/mol
H2= �D771.1 kJ/mol
O2PtF6 (s) = O2+ (g) + PtF6- (g)��![]() H3= 482.2 kJ/mol
H3= 482.2 kJ/mol
��ӦO2 (g) + PtF6 (g) = O2PtF6 (s)��![]() H=_____________ kJ/mol��
H=_____________ kJ/mol��
�����г����µ�������Һ����0.01 mol/L CH3COOH��Һ����0.01 mol/L HCl��Һ����pH=12�İ�ˮ����pH=12��NaOH��Һ����0.01 mol/L CH3COOH��Һ��pH=12�İ�ˮ�������Ϻ�������Һ����0.01 mol/L HCl��Һ��pH=12��NaOH��Һ��������������Һ��
(1)�ݡ�����Һ�Ƚϣ�pH�ϴ���� ��
(2)������Һ�У�ˮ�ĵ���̶���ͬ����______________��
(3)���ڡ��ۻ�Ϻ�������ҺpH=7����������Һ���������________ ��(ѡ�����������������)��
(4)ϡ����ͬ��������Һ��pH���� �ڣ��� ��(ѡ�����������������)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com