
| m |
| M |
| m |
| M |
| 37.6g |
| 188g/mol |
| 18.8g |
| 188g/mol |


 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��C4H10 |
| B��C2H2 |
C��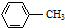 |
| D��CH3COOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� п�̵�� |
B�� ��ȼ�ϵ�� |
C�� Ǧ���� |
D�� ���ӵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
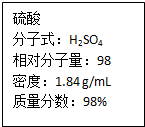 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
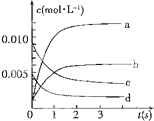 ��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO����mol�� | 0.020 | 0.01 | 0.008 | 0.007 | 0.007 | 0.007 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����ѣ�CH3OCH3������Ϊ21���������ȼ�ϣ���δ������������͡�Һ������ú���Ȳ����������Ļ������ܣ���ҵ�Ʊ��������ڴ���Ӧ���У�ѹ��2.0��10.0Mpa���¶�230��280�棩�������з�Ӧ��
�����ѣ�CH3OCH3������Ϊ21���������ȼ�ϣ���δ������������͡�Һ������ú���Ȳ����������Ļ������ܣ���ҵ�Ʊ��������ڴ���Ӧ���У�ѹ��2.0��10.0Mpa���¶�230��280�棩�������з�Ӧ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com