
·ÖĪö £Ø1£©øł¾Ż·“Ó¦Ē°ŗóŌŖĖŲ»ÆŗĻ¼ŪÅŠ¶Ļ£»
£Ø2£©ÅŠ¶ĻŃõ»Æ»¹Ō·“Ó¦µÄŅĄ¾Ż£ŗŹĒ·ńÓŠ»ÆŗĻ¼ŪµÄ±ä»Æ£»ÖĆ»»·“Ó¦£ŗµ„ÖŹÓė»ÆŗĻĪļ·“Ӧɜ³ÉĮķĶāµÄµ„ÖŹŗĶ»ÆŗĻĪļµÄ»Æѧ·“Ó¦£¬¾Ż“Ė½ā“š£®
½ā“š ½ā£ŗ£Ø1£©A£®3H2+Fe2O3$\frac{\underline{\;øßĪĀ\;}}{\;}$2Fe+3H2OÖŠH»ÆŗĻ¼ŪÉżøߣ¬Fe»ÆŗĻ¼Ū½µµĶ£¬¹ŹAÕżČ·£»
B£®Fe+CuSO4ØTCu+FeSO4ÖŠFe»ÆŗĻ¼ŪÉżøߣ¬Cu»ÆŗĻ¼Ū½µµĶ£¬¹ŹBÕżČ·£»
C£®Ba£ØOH£©2+H2SO4ØTBaSO4”ż+2H2OÖŠĪŽŌŖĖŲ»ÆŗĻ¼ŪÉż½µ£¬¹ŹC“ķĪó£»
D£®NH4HCO3$\frac{\underline{\;\;”÷\;\;}}{\;}$NH3”ü+CO2”ü+H2O”üÖŠĪŽŌŖĖŲ»ÆŗĻ¼ŪÉż½µ£¬¹ŹD“ķĪó£»
¹Ź“š°øĪŖ£ŗAB£»
£Ø2£©ÓÉ£Ø1£©µĆŹōÓŚŃõ»Æ»¹Ō·“Ó¦ĪŖAB£¬ŌņAB¶¼ŹĒµ„ÖŹÓė»ÆŗĻĪļ·“Ӧɜ³ÉĮķĶāµÄµ„ÖŹŗĶ»ÆŗĻĪļµÄ»Æѧ·“Ó¦£¬ĖłŅŌ¶¼ŹōÓŚÖĆ»»·“Ó¦£¬¹Ź“š°øĪŖ£ŗAB£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éĮĖ Ńõ»Æ»¹Ō·“Ó¦ŅŌ¼°ÖĆ»»·“Ó¦µÄÅŠ¶Ļ£¬±Č½Ļ¼ņµ„£¬×¢Ņā»ł±¾øÅÄīµÄÕĘĪÕ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
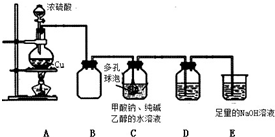
| ¼Ł Éč | ²Ł×÷ | ĻÖ Ļó | ŌĄķ |
| Na2S2O4 ĪŖĒæ ¼īČõĖįŃĪ£¬ĘäČÜ ŅŗĪŖ¼īŠŌ£® | ȔɣĮæČÜŅŗÓŚŹŌ¹ÜÖŠ£¬µĪ¼Ó ×ĻÉ«ŹÆČļŹŌŅŗ | ČÜŅŗ±ä ³ÉĄ¶É« | S2O42-Ė®½ā£¬Ź¹ČÜŅŗ³É¼īŠŌ |
| Na2S2O4 ÖŠ S ĪŖ +3 ¼Ū£¬¾ßÓŠ½ĻĒæ µÄ»¹ŌŠŌ£® | ȔɣĮæČÜŅŗÓŚŹŌ¹ÜÖŠ£¬µĪ¼Ó¹żĮæŠĀÖĘĀČĖ®£¬ŌŁ µĪ¼Ó BaCl2 ČÜŅŗ | ÓŠ°×É«³ĮµķÉś ³É | øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŅĄ“ĪĪŖ£ŗ 4H2O+S2O42-+3Cl2=2SO42-+6Cl-+8H+ Ba2++SO42-=BaSO4”ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 32g O2ÖŠŗ¬ÓŠ32NAøöµē×Ó | |
| B£® | 22.4L N2ŗ¬ÓŠ°¢·ü¼ÓµĀĀŽ³£ŹżøöµŖ·Ö×Ó | |
| C£® | ŌŚ±ź×¼×“æöĻĀ£¬22.4LĖ®µÄÖŹĮæŌ¼ĪŖ18g | |
| D£® | ³£ĪĀ³£Ń¹ĻĀ22gµÄCO2Óė±ź×¼×“æöĻĀ11.2L HClŗ¬ÓŠĻąĶ¬µÄ·Ö×ÓŹż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | ¢ŁÖŠŃō¼«“¦ÄܲśÉśŹ¹ŹŖČóµķ·ŪKIŹŌÖ½±äĄ¶µÄĘųĢå | |
| B£® | ¢ŚÖŠ“ż¶ĘĢśÖĘĘ·Ó¦ÓėµēŌ“Õż¼«ĻąĮ¬ | |
| C£® | ¢ŪÖŠøÖÕ¢ĆÅÓ¦ÓėĶā½ÓµēŌ“µÄÕż¼«ĻąĮ¬£¬³ĘĪŖĪžÉüŃō¼«µÄŅõ¼«±£»¤·Ø | |
| D£® | ¢ÜÖŠµÄĄė×Ó½»»»Ä¤æÉŅŌ±ÜĆāÉś³ÉµÄCl2ÓėNaOHČÜŅŗ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
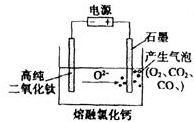 ČēĶ¼ĪŖEFC½£ĒÅ·ØÓĆ¹ĢĢ嶞Ńõ»ÆīŃ£ØTiO2£©Éś²śŗ£ĆąīѵÄ×°ÖĆŹ¾ŅāĶ¼£¬ĘäŌĄķŹĒŌŚ½ĻµĶµÄŅõ¼«µēĪ»ĻĀ£¬TiO2£ØŅõ¼«£©ÖŠµÄŃõ½āĄė½ųČėČŪŃĪ£¬Ņõ¼«×īŗóÖ»Ź£ĻĀ“æīŃ£®Ņõ¼«µÄµē¼«·“Ó¦Ź½ĪŖTiO2+4e-”śTi+2O2-£¬ŹÆÄ«µē¼«µÄÖŹĮæŹĒ·ń·¢Éś±ä»ÆŹĒ £ØĢī”°ŹĒ”±»ņ”°·ń”±£©£®
ČēĶ¼ĪŖEFC½£ĒÅ·ØÓĆ¹ĢĢ嶞Ńõ»ÆīŃ£ØTiO2£©Éś²śŗ£ĆąīѵÄ×°ÖĆŹ¾ŅāĶ¼£¬ĘäŌĄķŹĒŌŚ½ĻµĶµÄŅõ¼«µēĪ»ĻĀ£¬TiO2£ØŅõ¼«£©ÖŠµÄŃõ½āĄė½ųČėČŪŃĪ£¬Ņõ¼«×īŗóÖ»Ź£ĻĀ“æīŃ£®Ņõ¼«µÄµē¼«·“Ó¦Ź½ĪŖTiO2+4e-”śTi+2O2-£¬ŹÆÄ«µē¼«µÄÖŹĮæŹĒ·ń·¢Éś±ä»ÆŹĒ £ØĢī”°ŹĒ”±»ņ”°·ń”±£©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | MgF2µÄµē×ÓŹ½£ŗ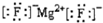 | B£® | ¶ž¼×ĆŃµÄ½į¹¹Ź½£ŗCH3-O-CH3 | ||
| C£® | NH3µÄĒņ¹÷Ä£ŠĶ£ŗ | D£® | ŃõŌ×ӵĽį¹¹Ź¾ŅāĶ¼£ŗ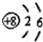 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ū¢Ż | B£® | ¢Ś¢Ü | C£® | ¢Ł¢Ś¢Ü | D£® | ¢Ś¢Ü¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2N2H4£Øg£©+N2O4£Øg£©ØT3N2£Øg£©+4H2O£Øg£©”÷H=-542.7 kJ/mol | |
| B£® | 2N2H4£Øg£©+N2O4£Øg£©ØT3N2£Øg£©+4H2O£Øg£©”÷H=-1 059.3 kJ/mol | |
| C£® | 2N2H4£Øg£©+N2O4£Øg£©ØT3N2£Øg£©+4H2O£Øg£©”÷H=+1 076.7 kJ/mol | |
| D£® | N2H4£Øg£©+$\frac{1}{2}$N2O4£Øg£©ØT$\frac{3}{2}$N2£Øg£©+2H2O£Øg£©”÷H=-538.35kJ/mol |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com