
ÓĆĖįŠŌKMnO4ŗĶH2C2O4£Ø²ŻĖį£©·“Ӧъ¾æÓ°Ļģ·“Ó¦ĖŁĀŹµÄŅņĖŲ£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗ2MnO4££«5H2C2O4£«6H£«£½2Mn2£«£«10CO2”ü£«8H2O”£Ņ»ŹµŃ銔×éÓūĶعż²ā¶Øµ„Ī»Ź±¼äÄŚÉś³ÉCO2µÄĖŁĀŹ£¬Ģ½¾æijÖÖÓ°Ļģ»Æѧ·“Ó¦ĖŁĀŹµÄŅņĖŲ£¬Éč¼ĘŹµŃé·½°øČēĻĀ£ØKMnO4ČÜŅŗŅŃĖį»Æ£©£ŗ
| ŹµŃéŠņŗÅ | AČÜŅŗ | BČÜŅŗ |
| ¢Ł | 20 mL 0.1 mol”¤L£1H2C2O4ČÜŅŗ | 30 mL 0.01 mol”¤L£1KMnO4ČÜŅŗ |
| ¢Ś | 20 mL 0.2 mol”¤L£1H2C2O4ČÜŅŗ | 30 mL 0.01 mol”¤L£1KMnO4ČÜŅŗ |

£Ø10·Ö£©
£Ø1£©ÅØ¶Č£Ø2·Ö£© ¢Ś£¾¢Ł£Ø2·Ö£©
£Ø2£©0.0092£Ø2·Ö£©
£Ø3£©KMnO4ČÜŅŗĶźČ«ĶŹÉ«ĖłŠčŹ±¼ä»ņ²śÉśĻąĶ¬Ģå»żĘųĢåĖłŠčµÄŹ±¼ä£Ø2·Ö£©
£Ø4£©øĆ·“Ó¦·ÅČČ£Ø2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©øł¾Ż±ķÖŠĖłĮŠŹĒŹż¾Ż·ÖĪö£¬ŹµŃéĢ½¾æµÄŹĒÅضČŅņĖŲ¶Ō»Æѧ·“Ó¦ĖŁĀŹµÄÓ°Ļģ”£ÅضČŌ½“ó·“Ó¦ĖŁĀŹŌ½æģ£¬Éś³ÉµÄCO2µÄĢå»żŌ½“󣬼“¢Ś£¾¢Ł”£
£Ø2£©4.48 mL CO2£¬¼“0.0002mol£¬øł¾Ż»Æѧ·½³ĢŹ½¼ĘĖć£¬·“Ó¦µÄMnO4£ŹĒ0.00004mol£¬Ź£ÓąµÄMnO4£ŹĒ0.0005mol-0.00004mol=0.00046mol£¬¹Źc(MnO4£)£½0.0092mol”¤L£1”£
£Ø3£©KMnO4ŹĒÓŠŃÕÉ«µÄ£¬¹Ź»¹æÉĶعż²ā¶ØKMnO4ČÜŅŗĶźČ«ĶŹÉ«ĖłŠčŹ±¼ä»ņ²śÉśĻąĶ¬Ģå»żĘųĢåĖłŠčµÄŹ±¼äĄ“±Č½Ļ»Æѧ·“Ó¦ĖŁĀŹ”£
£Ø4£©ÉżøßĪĀ¶Č»į¼Óæģ·“Ó¦µÄĖŁĀŹ”£
æ¼µć£ŗ»Æѧ·“Ó¦ĖŁĀŹµÄÓ°ĻģŅņĖŲ¼°¼ĘĖć
µćĘĄ£ŗ±¾Ģāæ¼²éĮĖÓ°Ļģ»Æѧ·“Ó¦ĖŁĀŹµÄŅņĖŲ¼°¼ĘĖć£¬ÄѶČÖŠµČ£¬×¢Ņā¾ö¶Ø·“Ó¦ĖŁĀŹµÄŹĒĪļÖŹ±¾ÉķµÄŠŌÖŹ£¬Ķā½ēĢõ¼žÖ»ŹĒÓ°ĻģŅņĖŲ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¢ń”¢£Ø8·Ö£©ÓŠŅ»Ń§ÉśŌŚŹµŃéŹŅ²āijČÜŅŗµÄpH”£ŹµŃ鏱£¬ĖūĻČÓĆÕōĮóĖ®ČóŹŖpHŹŌÖ½£¬Č»ŗóÓĆ½ą¾»øÉŌļµÄ²£Į§°ōõ“Č”ŹŌŃł½ųŠŠ¼ģ²ā”£
£Ø1£©øĆѧɜµÄ²Ł×÷ŹĒ”””””” ””””£ØĢī”°ÕżČ·µÄ”±»ņ”°“ķĪóµÄ”±£©”£
£Ø2£©Čē²»ÕżČ·£¬ŹĒ·ńŅ»¶ØÓŠĪó²ī£æ“š£ŗ””””””””””£ØĢī”°ŹĒ”±»ņ”°·ń”±£©
£Ø3£©Čō°““Ė·Ø·Ö±š²ā¶ØC£ØH£«£©ĻąµČµÄŃĪĖįŗĶ“×ĖįČÜŅŗµÄpH£¬Īó²ī½Ļ“óµÄŹĒ””””””””£¬ŌŅņŹĒ”””””””” ”””””””””£
¢ņ”¢£Ø10·Ö£©
£Ø1£©ČēĶ¼±ķŹ¾50mLµĪ¶Ø¹ÜÖŠŅŗĆęµÄĪ»ÖĆ£¬Ęä¶ĮŹżŹĒ mL
£Ø2£©ŅŅ¶žĖįĖ×Ćū²ŻĖį£¬Ä³»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ÓūĢ½¾æ²ā¶Ø
|
¢Ł”¢ĻĀĮŠŅĒĘ÷ŌŚ½ųŠŠµĪ¶ØŹ±£¬¾ų²»æÉŅŌŹĀĻČČóĻ“µÄŹĒ £ØĢī±ąŗÅ£©”£
¼×£®ĖįŹ½µĪ¶Ø¹Ü ŅŅ£®¼īŹ½µĪ¶Ø¹Ü ±ū£®25mLĮæĶ² ¶”£®×¶ŠĪĘæ
¢Ś”¢µĪ¶ØŹ±£¬½«KMnO4±ź×¼Ņŗ×°ŌŚ µĪ¶Ø¹ÜÖŠ”£
¢Ū”¢ ±¾ŹµŃéµĪ¶Ø“ļµ½ÖÕµćµÄ±źÖ¾æÉŅŌŹĒ
ӣ
¢Ü”¢ ČōµĪ¶ØÖÕµćŹ±ø©ŹÓµĪ¶Ø¹ÜæĢ¶Č£¬ŌņÓÉ“Ė²āµĆµÄxÖµ»į £ØĢī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğø£½ØŹ”ĘĪĢļŅ»ÖŠø߶žĻĀѧʌµŚŅ»Ń§¶Īæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
¢ń”¢£Ø8·Ö£©ÓŠŅ»Ń§ÉśŌŚŹµŃéŹŅ²āijČÜŅŗµÄpH”£ŹµŃ鏱£¬ĖūĻČÓĆÕōĮóĖ®ČóŹŖpHŹŌÖ½£¬Č»ŗóÓĆ½ą¾»øÉŌļµÄ²£Į§°ōõ“Č”ŹŌŃł½ųŠŠ¼ģ²ā”£
£Ø1£©øĆѧɜµÄ²Ł×÷ŹĒ”””””” ””””£ØĢī”°ÕżČ·µÄ”±»ņ”°“ķĪóµÄ”±£©”£
£Ø2£©Čē²»ÕżČ·£¬ŹĒ·ńŅ»¶ØÓŠĪó²ī£æ“š£ŗ””””””””””£ØĢī”°ŹĒ”±»ņ”°·ń”±£©
£Ø3£©Čō°““Ė·Ø·Ö±š²ā¶ØC£ØH£«£©ĻąµČµÄŃĪĖįŗĶ“×ĖįČÜŅŗµÄpH£¬Īó²ī½Ļ“óµÄŹĒ””””””””£¬ŌŅņŹĒ”””””””” ”””””””””£
¢ņ”¢£Ø10·Ö£©
£Ø1£©ČēĶ¼±ķŹ¾50mLµĪ¶Ø¹ÜÖŠŅŗĆęµÄĪ»ÖĆ£¬Ęä¶ĮŹżŹĒ mL
£Ø2£©ŅŅ¶žĖįĖ×Ćū²ŻĖį£¬Ä³»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ÓūĢ½¾æ²ā¶Ø
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģø£½ØŹ”ø߶žĻĀѧʌµŚŅ»Ń§¶Īæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
¢ń”¢£Ø8·Ö£©ÓŠŅ»Ń§ÉśŌŚŹµŃéŹŅ²āijČÜŅŗµÄpH”£ŹµŃ鏱£¬ĖūĻČÓĆÕōĮóĖ®ČóŹŖpHŹŌÖ½£¬Č»ŗóÓĆ½ą¾»øÉŌļµÄ²£Į§°ōõ“Č”ŹŌŃł½ųŠŠ¼ģ²ā”£
£Ø1£©øĆѧɜµÄ²Ł×÷ŹĒ”””””” ””””£ØĢī”°ÕżČ·µÄ”±»ņ”°“ķĪóµÄ”±£©”£
£Ø2£©Čē²»ÕżČ·£¬ŹĒ·ńŅ»¶ØÓŠĪó²ī£æ“š£ŗ””””””””””£ØĢī”°ŹĒ”±»ņ”°·ń”±£©
£Ø3£©Čō°““Ė·Ø·Ö±š²ā¶ØC£ØH£«£©ĻąµČµÄŃĪĖįŗĶ“×ĖįČÜŅŗµÄpH£¬Īó²ī½Ļ“óµÄŹĒ””””””””£¬ŌŅņŹĒ”””””””” ”””””””””£
¢ņ”¢£Ø10·Ö£©
£Ø1£©ČēĶ¼±ķŹ¾50mLµĪ¶Ø¹ÜÖŠŅŗĆęµÄĪ»ÖĆ£¬Ęä¶ĮŹżŹĒ mL
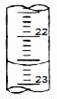
£Ø2£©ŅŅ¶žĖįĖ×Ćū²ŻĖį£¬Ä³»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ÓūĢ½¾æ²ā¶Ø
|
¢Ł”¢ĻĀĮŠŅĒĘ÷ŌŚ½ųŠŠµĪ¶ØŹ±£¬¾ų²»æÉŅŌŹĀĻČČóĻ“µÄŹĒ £ØĢī±ąŗÅ£©”£
¼×£®ĖįŹ½µĪ¶Ø¹Ü ŅŅ£®¼īŹ½µĪ¶Ø¹Ü ±ū£®25 mLĮæĶ² ¶”£®×¶ŠĪĘæ
¢Ś”¢µĪ¶ØŹ±£¬½«KMnO4±ź×¼Ņŗ×°ŌŚ µĪ¶Ø¹ÜÖŠ”£
¢Ū”¢ ±¾ŹµŃéµĪ¶Ø“ļµ½ÖÕµćµÄ±źÖ¾æÉŅŌŹĒ
ӣ
¢Ü”¢ ČōµĪ¶ØÖÕµćŹ±ø©ŹÓµĪ¶Ø¹ÜæĢ¶Č£¬ŌņÓÉ“Ė²āµĆµÄxÖµ»į £ØĢī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
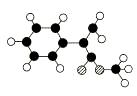 AŹĒÉś²śÄ³ŠĀŠĶ¹¤³ĢĖÜĮĻµÄ»ł“”ŌĮĻÖ®Ņ»£¬ÓÉC”¢H”¢OČżÖÖŌŖĖŲ×é³É£¬Ęä·Ö×Ó½į¹¹
AŹĒÉś²śÄ³ŠĀŠĶ¹¤³ĢĖÜĮĻµÄ»ł“”ŌĮĻÖ®Ņ»£¬ÓÉC”¢H”¢OČżÖÖŌŖĖŲ×é³É£¬Ęä·Ö×Ó½į¹¹
Ä£ŠĶČēĶ¼ĖłŹ¾£ØĶ¼ÖŠĒņÓėĒņÖ®¼äĮ¬Ļß“ś±ķ»Æѧ¼üµ„¼ü»ņĖ«¼ü£©”£
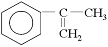
øł¾Ż·Ö×Ó½į¹¹Ä£ŠĶŠ“³öAµÄ·Ö×ÓŹ½____________ £¬A·Ö×ÓÖŠÖĮÉŁÓŠ________ øöĢ¼Ō×ÓŅ»¶Ø¹²Ę½Ćę£¬AµÄŗĖ“Ź²ÕńĒāĘ×ÓŠ_______øöĪüŹÕ·å”£
![]() £Ø2£©ŅŌ ĪŖÖ÷ŅŖŌĄķŗĻ³ÉAµÄĀ·ĻßČēĻĀ£ŗ
£Ø2£©ŅŌ ĪŖÖ÷ŅŖŌĄķŗĻ³ÉAµÄĀ·ĻßČēĻĀ£ŗ

(a)AµÄŗĻ³ÉĀ·ĻßÖŠŹōÓŚŃõ»Æ·“Ó¦µÄÓŠ__________ £ØĢīŠņŗÅ£©
(b)HµÄ½į¹¹¼ņŹ½ĪŖ___________
(c)Š“³ö·“Ó¦¢ŽµÄ»Æѧ·½³ĢŹ½£Ø×¢Ć÷±ŲŅŖµÄĢõ¼ž£©
______________________________________________________________________________________
(d)ŅŃÖŖ·“Ó¦¢ßĪŖĻūČ„·“Ó¦£¬ÓŠĶ¬Ń§ŹŌĶ¼ÓĆĖįŠŌKMnO4ČÜŅŗ½«FÖ±½ÓŃõ»Æ³ÉB£¬ÄćČĻĪŖøĆĻė·ØÄÜ·ńŹµĻÖ£æČōÄÜ£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬Čō²»ÄÜĒėĖµĆ÷ŌŅņ”£
_________________________________________________________________________________________
(e)GµÄijĶ¬·ÖŅģ¹¹Ģ壬±½»·ÉĻÖ»ÓŠŅ»øö²ąĮ“£¬ĒŅÄÜ·¢ÉśŅų¾µ·“Ó¦ŗĶĖ®½ā·“Ó¦£¬Š“³öĘäæÉÄܵĽį¹¹¼ņŹ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźÕć½Ź”µŚ¶ž“ĪĪ劣ĮŖæ¼ĄķæĘ×ŪŗĻŹŌĢā¾ķ ĢāŠĶ£ŗĢīæÕĢā
AŹĒÉś²śÄ³ŠĀŠĶ¹¤³ĢĖÜĮĻµÄ»ł“”ŌĮĻÖ®Ņ»£¬ÓÉC”¢H”¢OČżÖÖŌŖĖŲ×é³É£¬Ęä·Ö×Ó½į¹¹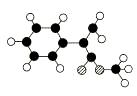
Ä£ŠĶČēĶ¼ĖłŹ¾£ØĶ¼ÖŠĒņÓėĒņÖ®¼äĮ¬Ļß“ś±ķ»Æѧ¼üµ„¼ü»ņĖ«¼ü£©”£
øł¾Ż·Ö×Ó½į¹¹Ä£ŠĶŠ“³öAµÄ·Ö×ÓŹ½____________ £¬A·Ö×ÓÖŠÖĮÉŁÓŠ________ øöĢ¼Ō×ÓŅ»¶Ø¹²Ę½Ćę£¬AµÄŗĖ“Ź²ÕńĒāĘ×ÓŠ_______øöĪüŹÕ·å”£
£Ø2£©ŅŌ ĪŖÖ÷ŅŖŌĄķŗĻ³ÉAµÄĀ·ĻßČēĻĀ£ŗ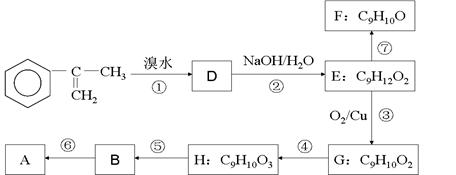
(a)AµÄŗĻ³ÉĀ·ĻßÖŠŹōÓŚŃõ»Æ·“Ó¦µÄÓŠ__________ £ØĢīŠņŗÅ£©
(b)HµÄ½į¹¹¼ņŹ½ĪŖ___________
(c)Š“³ö·“Ó¦¢ŽµÄ»Æѧ·½³ĢŹ½£Ø×¢Ć÷±ŲŅŖµÄĢõ¼ž£©
______________________________________________________________________________________
(d)ŅŃÖŖ·“Ó¦¢ßĪŖĻūČ„·“Ó¦£¬ÓŠĶ¬Ń§ŹŌĶ¼ÓĆĖįŠŌKMnO4ČÜŅŗ½«FÖ±½ÓŃõ»Æ³ÉB£¬ÄćČĻĪŖøĆĻė·ØÄÜ·ńŹµĻÖ£æČōÄÜ£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬Čō²»ÄÜĒėĖµĆ÷ŌŅņ”£
_________________________________________________________________________________________
(e)GµÄijĶ¬·ÖŅģ¹¹Ģ壬±½»·ÉĻÖ»ÓŠŅ»øö²ąĮ“£¬ĒŅÄÜ·¢ÉśŅų¾µ·“Ó¦ŗĶĖ®½ā·“Ó¦£¬Š“³öĘäæÉÄܵĽį¹¹¼ņŹ½
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com