
 2mL������Һ�������������ᱵ��Һ����������˲�����ϡ����İ�ɫ������
2mL������Һ�������������ᱵ��Һ����������˲�����ϡ����İ�ɫ������
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
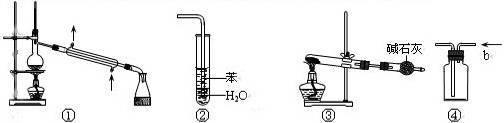
| A��װ�âٳ����ڷ��뻥���ܽ�е㲻ͬ��Һ������ |
| B��װ�âڿ���������HCl����������ˮ�����壬����ֹ���� |
| C����NaHCO3Ϊԭ�ϣ�װ�âۿ�����ʵ�����Ʊ�����CO2 [��Ϣ��ʾ��2NaHCO3=Na2CO3+CO2��+H2O����ʯ��Ϊ�ռ�����ʯ�ҵĻ����] |
| D��װ�â��У�b�ڽ������ռ�CO2��NO������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��FeSO4 (CuSO4)�����������ۺ��� |
| B��CO (CO2)����NaOH��Һϴ������� |
| C��MnO2 (KCl)����ˮ�ܽ���ˡ�ϴ�ӡ���� |
| D��CO2 (HCl)����NaOH��Һϴ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ܢ٢ۢ� | B���ܢڢۢ� | C���ۢڢܢ� | D���ڢۢ٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����ȡ��Һ�� | B���ᾧ�� | C����Һ�� | D������ E�����˷� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A���ƾ���ˮ | B��������Ȼ�̼ |
| C��ˮ�����Ȼ�̼ | D�����ͺ�ֲ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2O��AgNO3��Һ��ϡHNO3 |
| B��H2O��KOH��Һ������ |
| C��H2O��KOH��Һ��ϡHNO3 |
| D��H2O��AgNO3��Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ������÷�Һ©�����з������
������÷�Һ©�����з������| A��ˮ��ƾ� | B��ˮ��ֲ���� | C��ˮ�����  | D��ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com