
���и���������ָ���������ܴ����������(����)
��Ư�۵�ˮ��Һ�У�Fe2����Cl����Ca2����Na�����ڵμ�ʯ����Һ�ʺ�ɫ����Һ��K����NH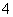 ��Cl����S2�������ܹ������Cu�����·�Ӧ�ų��������Һ��Fe3����Al3����SO
��Cl����S2�������ܹ������Cu�����·�Ӧ�ų��������Һ��Fe3����Al3����SO ��K��
��K��
�ܳ�����pH��2����Һ�У�NH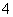 ��Na����Cl����Cu2��
��Na����Cl����Cu2��
����ɫ��Һ�У�K����CH3COO����HCO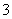 ��MnO
��MnO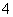
A���ڢ� B���٢�
C���ۢ� D���٢�
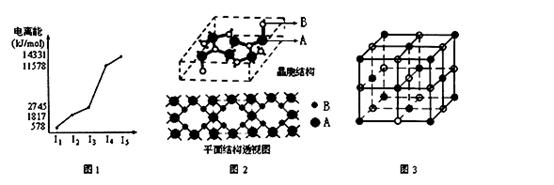
���������⿼�����ӹ��棬���ڿ��鿼�������ӷ�Ӧ���������������Ӧ����������Ư�۵�ˮ��Һ�е�ClO����Fe2������������ԭ��Ӧ�����ܴ������棻�ڵμ�ʯ����Һ�ʺ�ɫ����ҺΪ������Һ��S2�����ܴ������ڣ����ܹ������Cu�����·�Ӧ�ų��������Һ�к������ᣬ���е����ӿɴ������棻��pH��2����ҺΪǿ������Һ�����е����ӿɴ������棻��MnO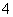 ����ɫ������ɫ��Һ�в��ܴ������ڡ�����������C����ȷ��
����ɫ������ɫ��Һ�в��ܴ������ڡ�����������C����ȷ��
�𰸣�C



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y�Ƕ�����Ԫ�أ���������ɻ�����X2Y3����֪Xԭ������Ϊn����Y��ԭ��������������
A��n��11 B��n��3 C��n��5 D��n��6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������CO2ͨ��KOH��Ca(OH)2�Ļ��ϡ��Һ�У����ɳ��������ʵ���(n)��ͨ��CO2���(V)�Ĺ�ϵ��ȷ���� (����)

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ѧ����ѡ��3���ʽṹ�����ʡ���15�֣�
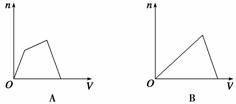
A��B��C��D��E��FΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ���������������Ҿ���ͬ�塣A��B��C����Ԫ�صĻ�̬ԭ�Ӿ�����ͬ���ܲ���ܼ����ҵ�һ������I1(A)��I1(C)��I1(B)��BC2+��AC2��Ϊ�ȵ����壻D��EΪͬ��������Ԫ�أ�FԪ��λ�����ڱ���1��18���еĵ�11�С���ش��������⣨����ʱ������Ӧ��Ԫ�ط��ű�ʾ��ӦԪ�أ���
��AԪ�صļ۲�����Ų�ͼΪ ��BC2+�ĵ���ʽΪ �� ��̬��ԭ�ӵĺ�������Ų���ʽ ��
��EԪ�صĵ����������ͼ1��ʾ��EԪ�������ڱ���λ�� �� ��Ԫ�ص����γɵ�
���徧���������� �ѻ���
��A��B��Ԫ�����γɵĻ������������һָ�ij�Ӳ�²��ϣ��侧���ṹ��ͼ2��ʾ���ɴ˿�֪��������
�ľ�������Ϊ ����Ӳ�ȳ������ʯ��ԭ���� ��������ľ�����Bԭ�ӵ��ӻ���ʽΪ ��
��D��C��Ԫ�ؿ��γɵĻ������ҡ�
����֤ʵ�������ҵľ���ṹ��ͼ3��ʾ��������D���ӵ���λ��Ϊ ��
����D���Ӱ뾶�ֱ�Ϊa��C���Ӱ뾶�ֱ�Ϊb�����侧��Ŀռ�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ȷ����(����)
| A | B | C | D |
|
|
|
|
|
| ����ʱ�ᷢ����Ӧ��N2��O2 | ���ö����ЧӦ֤���ձ��еķ�ɢϵ�ǽ��� | �����м���Ũ���������ڣ���ΪŨ��������ˮ�� | ����ʱ�ۻ����������䣬֤��Al2O3���۵��Al�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и�������ָ������Һ��һ���ܴ����������(����)
A���������þ����H2����Һ�У�NH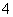 ��Na����SO
��Na����SO ��CH3COO��
��CH3COO��
B�����д���ClO������Һ�У�K����Na����NO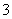 ��SO
��SO
C��c(Cu2��)��0.1 mol��L��1����Һ�У�H����NH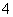 ��Br����CO
��Br����CO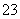
D�����д���MnO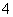 ����Һ�У�H����SO
����Һ�У�H����SO ��Cl����CH3CH2OH
��Cl����CH3CH2OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ͨ�����и����ʵ���Һ�У������Ӻ������Ӷ��ܱ��������� (����)
A��NaOH B��Na2SO3 C��FeBr2 D��FeSO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)(3)]����(COCl2)�����ϡ��Ƹ��ҩ�ȹ�ҵ����������;����ҵ�ϲ��ø�����CO��Cl2�ڻ���̿���ºϳɡ�
(1)ʵ�����г������Ʊ������Ļ�ѧ����ʽΪ_________________________________��
(3)ʵ�����п����ȷ�(CHCl3)��˫��ˮֱ�ӷ�Ӧ�Ʊ��������䷴Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com