
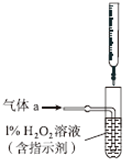
 ���Ṥҵ�����ķ�������Ҫ�ɷ֣�SO2��O2��N2��CO2�ȣ��ŷŵ������л���Ⱦ������ij��ѧ��ȤС��Է�������ɽ���̽������ش��������⣺
���Ṥҵ�����ķ�������Ҫ�ɷ֣�SO2��O2��N2��CO2�ȣ��ŷŵ������л���Ⱦ������ij��ѧ��ȤС��Է�������ɽ���̽������ش��������⣺
���� �������ʵ��������A�к��е�CO2����Ҫ��ȥ������������Ŷ�����̼�ļ��飬�����������������ͨ������ʯ��ˮ���������̼�Ĵ��ڣ�
������1��˫��ˮ����ǿ�����ԣ�����������л�ԭ�ԣ����߷���������ԭ��Ӧ���������ˮ��
��2��������1mL NaOH��Һ�൱���������Ϊy�ˣ�z mL NaOH��Һ�൱���������Ϊyzg���ٸ�����Ԫ���غ���������������������
�����ҷ������õ�ԭ��Ϊ��SO2+H2O2=H2SO4��H2SO4+Ba��OH��2=BaSO4��+2H2O�����������ᱵ����������β���ж����������������������β��������������
��1�������������������������Ӧ�������ᱵ��ˮ��
��2��ϴ�ӳ����ķ����ǣ���©����ע������ˮ��ʹˮ��û���������ˮ�������ظ�����2��3�Σ�
���������������в����ʡ�ԣ�ֱ�ӽ�β��ͨ�����Ba��OH��2��Һ��BaSO3����������ΪBaSO4��
��� �⣺�������Ṥҵ�����ķ���A����Ҫ�ɷ֣�SO2��O2��N2��CO2�ȣ��ŷŵ������л���Ⱦ���������ʵ���������к��е�CO2����Ҫ��ȥ������������Ŷ�����̼�ļ��飬�����������������ͨ������ʯ��ˮ���������̼�Ĵ��ڣ�ʯ��ˮ�����֤�����ж�����̼������ѡ���Լ�Ϊ���������Һ��ȥ��������ѡ�����ʯ��ˮ���������̼�Ĵ��ڣ��۲쵽��ʵ�������Ǹ��������Һ����ɫ��������ʯ��ˮ����ǣ�֤����������̼��
�ʴ�Ϊ��BC�����������Һ����ɫ��������ʯ��ˮ����ǣ�
����1��˫��ˮ����ǿ�����ԣ�����������л�ԭ�ԣ����߷���������ԭ��Ӧ���������ˮ����Ӧ����ʽΪ H2O2+SO2=H2SO4���ʴ�Ϊ��H2O2+SO2=H2SO4��
��2��������1mL NaOH��Һ�൱���������Ϊy�ˣ�z mL NaOH��Һ�൱���������Ϊyzg�������¶����������������ı���ʽ=$\frac{\frac{yzg}{32g/mol}��22.4L/mol}{aL}$��100%��
�ʴ�Ϊ��$\frac{\frac{yzg}{32g/mol}��22.4L/mol}{aL}$��100%��
�����ҷ������õ�ԭ��Ϊ��SO2+H2O2=H2SO4��H2SO4+Ba��OH��2=BaSO4��+2H2O�����������ᱵ����������β���ж����������������������β��������������
��1��������з�Ӧ�����ӷ���ʽΪ��2H++SO4+Ba2++2OH-=BaSO4��+2H2O���ʴ�Ϊ��2H++SO4+Ba2++2OH-=BaSO4��+2H2O��
��2��ϴ�ӳ����ķ����ǣ���©����ע������ˮ��ʹˮ��û���������ˮ�������ظ�����2��3�Σ�
�ʴ�Ϊ����©����ע������ˮ��ʹˮ��û���������ˮ�������ظ�����2��3�Σ�
���������������в����ʡ�ԣ�ֱ�ӽ�β��ͨ�����Ba��OH��2��Һ��BaSO3����������ΪBaSO4�����²ⶨ�������ᱵ������ƫ�ⶨ������������ƫ���������ƫ�ʲ�������
�ʴ�Ϊ����������BaSO3����������ΪBaSO4��
���� ���⿼��ѧ����ʵ��ԭ����ʵ����������⡢ʵ�鷽����ơ�Ԫ�ػ��������ʡ���ѧ����ȣ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | AgCl��AgBr��AgI����������ˮ�Ĺ��壬��Ҫ�ܹⱣ������ɫƿ�� | |
| B�� | AgCl�ǰ�ɫ������ˮ�Ĺ��壬����ָ�Ƽ��������ھ����ư� | |
| C�� | AgBr�Ǻ�ɫ���������ʣ������������������˹����� | |
| D�� | AgI�ǻ�ɫ���壬�������ֽ�Ϊ���ʵ�ͺ�ɫ�ĵ����������������˹����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
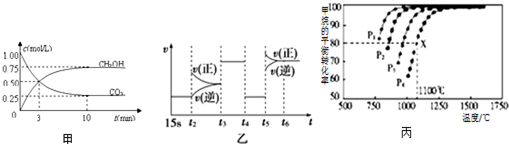
| T��K�� | K1 | K2 |
| 973 | 1.47 | 2.36 |
| 1173 | 2.15 | 1.67 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHCO3��Һ�м������CO32-+2H+�TCO2��+H2O | |
| B�� | ��ɫʯ����Һ�е������������ԭ���ǣ�H2S?2H++S2- | |
| C�� | �����ʵ�����MgCl2��Ba��OH��2�� HC1��Һ��ϣ�Mg2++2OH-�TMg��OH��2�� | |
| D�� | Ǧ�����س��ʱ��������Ӧ��PbSO4+2H2O-2e-�TPbO2+4H++SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
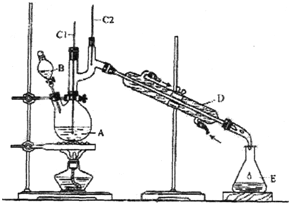 ����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ��
����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ��| �е�/�� | �ܶ�/��g•cm-3�� | ˮ���ܽ��� | |
| ������ | 117.2 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCl | B�� | BaCl2 | C�� | NaOH | D�� | AgNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2x | B�� | 4 | C�� | $\frac{y}{2}$ | D�� | 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | N��O��Fԭ�������������������� | |
| B�� | N��O��Fԭ�Ӱ뾶�������� | |
| C�� | Na��Mg��AlԪ����������ϼ��������� | |
| D�� | Li��Na��K�Ľ�����������ǿ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com