
���� ��1����Cr2O72-�����Թ�ҵ��ˮ�м�������������Ӧ����Fe3+��Cr3+��ˮ������ԭ���غ�͵���غ���ƽ��д���ӷ���ʽ��
��ʵ���Ҵ��Բⶨ��ҺpH�ķ�������pH��ֽ���ڽྻ�ı������ϣ��ò�����պȡ��Һ������pH��ֽ�ϣ��������ɫ�����գ�
��2���ɷ���ʽ�ɵù�ϵʽ2Cr��Cr2O72-��6I-��6S2O32-�����ݹ�ϵʽ����CrԪ������������ˮ�и�Ԫ����Ũ��=$\frac{m��Cr��}{V}$���м��㣮
��� �⣺��1������Cr2O72-�����Է�ˮ�м���FeSO4��Һ��ʹCr2O72-ȫ��ת��ΪCr3+���������ӱ�����Ϊ�����ӣ���Ӧ�����ӷ���ʽΪ��Cr2O72-+6Fe2++14H+=2Cr3++6Fe3++7H2O��
�ʴ�Ϊ��Cr2O72-+6Fe2++14H+=2Cr3++6Fe3++7H2O��
��ʵ���Ҵ��Բⶨ��ҺpH�ķ���Ϊ����pH��ֽ���ڽྻ�ı������ϣ��ò�����պȡ��Һ������pH��ֽ�ϣ��������ɫ�����գ�
�ʴ�Ϊ����pH��ֽ���ڽྻ�ı������ϣ��ò�����պȡ��Һ������pH��ֽ�ϣ��������ɫ�����գ�
��2���ɢ�2Cr3++3S2O82-+7H2O�TCr2O72-+6SO42-+14H+
��Cr2O72-+6I-+14H+�T2Cr3++3I2+7H2O
��I2+2S2O32-�T2I-+S4O62-
�ɵù�ϵʽ��2Cr��Cr2O72-��6I-��6S2O32-��
n��Na2S2O3��=20.00mL��0.015mol/L=3��10-4mol
��n��Cr��=1��10-4mol
m��Cr��=1��10-4mol��52g•mol-1=5.2��10-3 g=5.2mg
��ˮ�и�Ԫ����Ũ��=$\frac{5.2mg}{0.025L}$=208 mg•L-1
�𣺷�ˮ�и�Ԫ����Ũ��Ϊ208 mg•L-1��
���� ���⿼��������ԭ��Ӧ����ʽ����д��������ԭ��Ӧ�ζ�����ȣ��Ѷ��еȣ���2��ע�����ù�ϵʽ���еļ��㣮


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 11.7g | B�� | 23.4 g | C�� | 26.5g | D�� | 58.5g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH3 | B�� | O3 | C�� | CO2 | D�� | BeCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
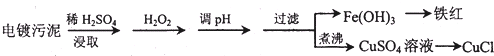
| ������ | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| ��ʼ������pH | 2.3 | 7.6 | 4.4 |
| ��ȫ������pH | 3.2 | 9.7 | 6.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ʵ�����ʵ����������ж���ȷ���ǣ�������
��ʵ�����ʵ����������ж���ȷ���ǣ�������| A�� | ʵ��������ɫ���� | B�� | ʵ�����Һ��ɫ��� | ||
| C�� | ʵ��ų��������� | D�� | ʵ������ȳ��ְ�ɫ���������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | n1��n2 | B�� | n1=n2 | C�� | n2��n1 | D�� | c��F-����c��CN-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ڵ���ƽ�� | B�� | ��������Ŀǰ�ߴ��ں��� | ||
| C�� | c��OH-��ǰ��С�ں��� | D�� | ���ڵ�����������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���߶�Ϊs-s �� �� | B�� | ���߶�Ϊp-p �� �� | ||
| C�� | ǰ��Ϊp-p �� ��������Ϊs-p �� �� | D�� | ǰ��Ϊs-s �� ��������Ϊs-p �� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO2��ˮ��Һ�ܵ��磬����SO2�ǵ���� | |
| B�� | ��ʽ�ε�ˮ��Һ������ | |
| C�� | ������Һ�д���ĵ���̶����¶ȵ����߶����� | |
| D�� | FeCl3����Һ�������ɿɵõ�FeCl3�ľ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com