
ij��ѧ����С���о��Ҵ�������ʵ�鲢��֤��������˼ס��Ҷ���װ�ã�ͼ�еļг�������δ��������������ʾ�ƾ�����Դ����ÿ��װ���ֿɻ���Ϊ�١��ڡ��������֡�������ʢ�ŵ��Լ�Ϊ��a����ˮ�Ҵ����е㣺78 �棩��b��ͭ˿��c����ˮ����ͭ��d��������Һ��
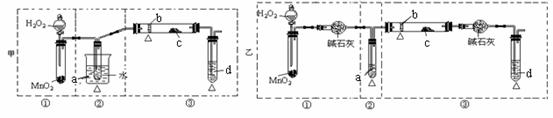
��1���������������Ե��ŵ㣺
��
��
��2���������������ŵ㣬���һ�ױȽϺ������Ƶ�ʵ��װ�ã��ɰ������������ҵ�˳���ʾΪ________________ ������ע٣��Ңڣ�
��3����Ҫ��֤��ʵ���нϸߵ�Ч�ʣ����貹��������� ������ ��
��4��д��d�еĻ�ѧ����ʽ ���÷�Ӧ����Ϊ ��
��5��װ���У�����ȥ�ڢٲ��֣������������䣬����ˮ����ͭ�����Ա仯�����������루4����ͬ���ƶ�ȼ�չ�����Ҫ��Ӧ�Ļ�ѧ����ʽ_______________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


 [AgI2]-
[AgI2]-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ش��������⣺?
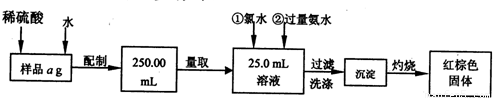
(1)��м��������������(��Ҫ�ɷ�ΪFe2O3��aH2O)����ȡ������������Ӱ�� (��С����ޡ�)����ԭ���ǣ� ������Ӱ�콫��β�������������(����Ӱ�죬���ʿ��Բ���)��
(2)FeSO4�ڲ�ͬ�¶��µ��ܽ�����±���ʾ��
�¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
�ܽ��/g | 18.5 | 20.8 | 26.3 | 32.8 | 40.1 | 48.4 | 52.4 | 50.9 | 43.7 |
���ڷ���м����C��Si�����ʣ�Ϊ��ʹFeSO4��Һ��Ũ�������ڷ�Ӧ�������Һ�Ͳ�������С��ʵ��ʱ��ȡ��ˮԡ���Ⱥͳ��ȹ��˵ķ���������������ҪĿ����Ϊ�˷�ֹ��������������Ϊʵ��ʱ��ˮԡ�¶���ÿ���������������?���ҡ�??
(3)�ⶨ�����нᾧˮ�������õ�ʵ��������������ƽ(��������ƽ)���в�������������ǯ�����żܡ������ǡ��ƾ��ơ�ҩ���⣬���������������жϾ����нᾧˮ��ȫʧȥ��ʵ�����������������?
(4)���������������廯ѧʽ��x��ʵ��ֵ����ʽΪx=��������?��(��֪���������������нᾧˮ������Ϊm g�������������������Ϊw g)?
(5)������м����˿�����ᷴӦ��������ʵ��������е�ͬѧ������õ��ķ��������Լӿ���ȡFeSO4��Һ�ķ�Ӧ���ʣ��÷����ĵ������Һ�����������������ĵ缫��Ӧ����ʽΪ���� ��?
������д��һ��(�������ı��¶Ⱥ������Ũ��)��������Ϊ��Ӧ��ܼӿ���ȡFeSO4��Һ��Ӧ���ʵķ���������������?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ����С��Ϊ��̽����ͬ������������ֽ����ʵ�Ӱ�죬��Ʋ�����������ʵ�飬����������и�������
I������ͭ���Ʊ���
��1����ȡ10g�������������С�ձ��У�����������ˮ�����Һ��
��2�����裨1���е��ձ��ڵμ�NaOH��Һ��ֱ�����������ij�����
��3����������Ƿ���ȫ������������� ��
��4�������裨2�����û�������������ȫ����Ϊ��ɫ��
��5���ٽ����裨4�����û���� ��ϴ�ӡ� ����ϸ����������Ҫ��֤�Ƿ�ϴ����������ӵķ����ǣ� ��
II���Ƚϲ�ͬ�����Թ�������ֽ����ʵ�Ӱ�졣
�ÿ���С������ɵ�ʵ�鼰ʵ���¼�ı������£�
�������ͼ��ʾװ�����ⶨ�����������

| ʵ����� | ˫��ˮ��� | ���� | �������� |
| �� | 15mL | �� | |
| �� | 15mL | CuO(0.5g) | |
| �� | 15mL | MnO2(0.5g) |
�Իش��������⣺
��1����ʵ����Ӱ��˫��ˮ�ֽ����ʵ������У���ʵ��ʱ���¶Ⱥ�ѹǿ����˫��ˮ��Ũ��
�۲�ͬ�Ĵ������� ���� �ȡ�
��2������ʵ���еġ��������ݡ������� ��Ҳ������ ��
��3��Ϊ̽��CuO��ʵ������Ƿ�������ã�����ٱȽ��⣬���貹������ʵ�飨����д�������������֤��CuO�Ļ�ѧ����û�иı䣻�� ��
��4������Ϊ����ʹ��������ֽ�Ĵ������� ����һ�����ʵĻ�ѧʽ�����ƣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ٳ���CuSO4��5H2Om1g�����в�����ϸ����ȫ��������ˮ�У�
����ʢ����ͭ��Һ���ձ��ڼ����Թ�����NaOH��Һ��ʹCu2+��ȫ������
�۽��ձ��ڵ�����Һת�Ƶ��������У�С��������У����裬ֱ��������ȫ��Ϊ��ɫ��
����ȴ���ˣ���������ˮϴ�ӳ���2��3�Σ�
�ݽ������ָ��������CuO m2g����ϸ���á�
��ش�
(1)��ʵ�����õ��������������÷ֱ�Ϊ�����___________�������___________�������___________��
(2)�������֤��NaOH�������ķ�����_________________��
(3)�������ϴ�ӳ����IJ���Ϊ_________________��Ŀ����___________________��
��.��С���ð�����ԭ����ͭ�ķ����ⶨͭ�Ľ������ԭ����������Ӧ�Ļ�ѧ����ʽΪ��
2 NH3+3CuO![]() N2+3Cu+3H2O
N2+3Cu+3H2O
��ش�
(1)���ѡ�òⶨ������Cu��H2O������m(Cu)��m(H2O)ʱ�����������������һ����ʵ�鷽����

���������ӵ�˳��(����ĸ��ű�ʾ���������ظ�ʹ��)_____________��d��������___________��b���Լ�X��������___________��
���г�����Cu�����ԭ�������ı���ʽ___________��
(2)������Բ�����������װ�ã��ⶨ����������Ҳ���Դﵽʵ��Ŀ�ĵ���___________��
A.m(CuO)��m(H2O) B.V(N2)��m(CuO)
C.m(NH3)��m(H2O) D.m(CuO)��m(Cu)
����������������װ�ã�Ϊ�ﵽʵ��Ŀ�ģ�����A��B��C��D������ѡ��___________����ɴ�ѡ���ʵ�飬Ӧ�����ӵ�������___________(ѡ��װ�ô���)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���Ĵ�ʡ�ɶ���˫���ظ߿���ѧģ���Ծ��������棩 ���ͣ������
 [AgI2]-
[AgI2]-
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com