
���� �ٽ����ַ�ĩ����ˮ�У���Һ����ɫ��˵��һ��������CuSO4�����а�ɫ�������ɣ�˵��һ������Ba��NO3��2��Na2CO3����̼�ᱵ��ɫ������
����ٵij������м�������ϡ���ᣬ������ȫ�ܽ⣬�������ݲ�����֤����̼�ᱵ������
��ȡ�����ڵ���Һ������ϡ���ᣬ�а�ɫ����������֤�����ɵ������ᱵ������
����ȡ���й��˺����Һ��������AgNO3��Һ��������ɫ������һ����̼�������������ܺ����Ȼ����������Դ������
��� �⣺���������Ϣ��ʵ����̿�֪��
�ٽ����ַ�ĩ����ˮ�У���Һ����ɫ��˵��һ��������CuSO4�����а�ɫ�������ɣ�˵��һ������Ba��NO3��2��Na2CO3����̼�ᱵ��ɫ������
����ٵij������м�������ϡ���ᣬ������ȫ�ܽ⣬�������ݲ�����֤����̼�ᱵ������
��ȡ�����ڵ���Һ������ϡ���ᣬ�а�ɫ����������֤�����ɵ������ᱵ������
����ȡ���й��˺����Һ��������AgNO3��Һ��������ɫ������һ����̼�������������ܺ����Ȼ���������
��1������������֪һ������Ba��NO3��2��Na2CO3��һ������CuSO4�����ܺ���NaCl���ʴ�Ϊ��Ba��NO3��2��Na2CO3��CuSO4��NaCl��
��2�����ݸ���ʵ�������֪��
�ٹ����ϵõ���ɫ��������ɫ��Һ��һ��������CuSO4�����ɳ�������Ba��NO3��2��Na2CO3�����ɵ�̼�ᱵ��������Ӧ�����ӷ���ʽΪ��Ba2++CO32-=BaCO3�����ʴ�Ϊ��Ba2++CO32-=BaCO3����
����ٵij������м�������ϡ���ᣬ������ȫ�ܽ⣬�������ݲ�������̼�ᱵ��������ϡ�������ɶ�����̼���壬��Ӧ�����ӷ���ʽΪ��BaCO3+2H+=Ba2++CO2��+H2O���ʴ�Ϊ��BaCO3+2H+=Ba2++CO2��+H2O��
��ȡ�����ڵ���Һ������ϡ���ᣬ�а�ɫ����������֤�����ɵ������ᱵ��������Ӧ�����ӷ���ʽΪ��Ba2++SO42-=BaSO4�����ʴ�Ϊ��Ba2++SO42-=BaSO4����
����ȡ���й��˺����Һ��������AgNO3��Һ��������ɫ�����������ƶϿ�֪һ������̼���ƣ�����һ����̼�������������ܺ����Ȼ�����������Ӧ�����ӷ���ʽΪ��2Ag++CO32-=Ag2CO3����Ag++Cl-=AgCl�����ʴ�Ϊ��2Ag++CO32-=Ag2CO3����Ag++Cl-=AgCl����
��3��ȡ�ٵõ�����ɫ����Һ�������Թ��У���������HNO3��Һ���ٵμ�����AgNO3��Һ��������ɫ�������˳���һ��ΪAgCl�����ų���̼�����ĸ��ţ���ôԭ��ĩ��һ������NaCl���ʴ�Ϊ��һ������NaCl��
���� ���⿼�����������ʵ�ʵ�������жϣ����ʼ����ʵ�鷽���ķ���ϢӦ�ã������������ʺ�������Ӧ�����ǽ���ؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڳ�ʪ�������������� | B�� | �ڸ������ܲ����������� | ||
| C�� | �ں���Ԫ�ؽ϶������������ | D�� | �ں�̼���϶࣬��ʪ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͭƬ����ϡ�����У�Cu+2H+=Cu2++H2�� | |
| B�� | ����μ���ʯ��ʯ�ϣ�CO32-+2H+=H2O+CO2�� | |
| C�� | ����������Һ��ϡ�����ϣ�Ba2++SO42-+H++OH-=BaSO4��+H2O | |
| D�� | ����ͭ��Һ������������Һ��Ӧ��Ba2++SO42-+Cu2++2 OH-=BaSO4��+Cu��OH��2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | Pb2+ | Ca2+ | Fe3+ | Mn2+ | Cl- |
| ����ǰŨ�ȣ�mg/L�� | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
| ������Ũ�ȣ�mg/L�� | 0.004 | 22.6 | 0.040 | 0.053 | 49.8 |
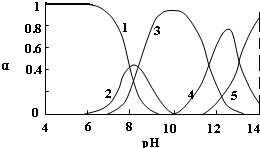
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
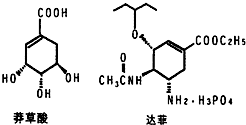 Ŀǰ��������֤������ơ������������кͼ���H1N1���е�����������ơ�����Ҫ�ϳ�ԭ�����ҹ�ʢ����ç���ᣮ�Ӱ˽���������ȡ��ç���ᾭ����η�Ӧ�����������Ƴɡ���ơ���������ͼ��ç����͡���ơ���Ч�ɷֵļ���ʽ�ṹ��Ш��ʵ�ߡ����߱�ʾ���ŵ�����ṹ����
Ŀǰ��������֤������ơ������������кͼ���H1N1���е�����������ơ�����Ҫ�ϳ�ԭ�����ҹ�ʢ����ç���ᣮ�Ӱ˽���������ȡ��ç���ᾭ����η�Ӧ�����������Ƴɡ���ơ���������ͼ��ç����͡���ơ���Ч�ɷֵļ���ʽ�ṹ��Ш��ʵ�ߡ����߱�ʾ���ŵ�����ṹ����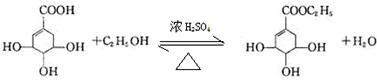 ��
��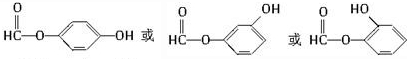 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ������������ķ�Ӧ Fe+2 H+=Fe2++H2�� | |
| B�� | ��ˮ�����ᷢ������кͷ�Ӧ OH-+H+=H2O | |
| C�� | ͭƬ������������Һ��Cu+Ag+=Cu2++Ag | |
| D�� | ̼��Ƽ��������Һ�� CaCO3+2CH3COOH=CO2��+2CH3COO-+H2O+Ca2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com