
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ�IJ�������ѡ��ʹ�õ�������û��450mL�������ƿ������ѡ��500mL����ƿ��
��2������n=CV��m=nM�����㣻
��3����������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ�������
��4������C=$\frac{n}{V}$��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
��� �⣺��1��û��450mL�������ƿ������ѡ��500mL����ƿ��������Һ�IJ������裺���ȼ������Ҫ��ҩƷ��������Ȼ����������ƽ������������ձ����ܽ⣬ͬʱ�ò��������裬����Һ��ȴ�����º��ò�����������Һ��500ml����ƿ��Ȼ��ϴ���ձ��Ͳ�����2��3�Σ���ϴ��ҺҲע������ƿ��Ȼ��������ƿ��עˮ����Һ����̶���1��2CMʱ�����ý�ͷ�ι���μ��룬����Һ����̶������У�Ȼ��ҡ�ȡ�װƿ��ʵ��������õ��������У�������ƽ���ձ�����������500ml����ƿ����ͷ�ιܣ��ʱ����õ��IJ��������У��ձ�����������500ml����ƿ����ͷ�ιܣ�
�ʴ�Ϊ���ձ�����������500ml����ƿ����ͷ�ιܣ�
��2��������450ml������ƿ����ѡ��500ml������ƿ�������Ƶ���500ml0.1mol/LNaOH��Һ������n=CV��֪��Ҫ��NaOH�����ʵ���n=0.5L��0.1mol/L=0.05mol��
����m=nM=0.05mol��40g/mol=2.0g��
�ʴ�Ϊ��2.0g��
��3�����Ʋ����м��㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ��������ƹ���������IJ����Ⱥ�˳��Ϊ��BCAED��
�ʴ�Ϊ��BCAED��
��4��A���������������ƫ��m��=m��+m�����ʳ�������ҩƷ������ƫ�أ������Ƴ�����Һ��Ũ��ƫ��
B����NaOH����ֽ���ϳ����ᳱ�⣬����������NaOH������ƫС�������Ƴ�����Һ��Ũ��ƫС��
C��NaOH���ձ����ܽ��δ��ȴ��ת�Ƶ�����ƿ�в����ݣ�����Һ��ȴ�����ƫС����Ũ��ƫ��
D��������ƿת��ʱ��������Һ�彦�����ᵼ�����ʵ���ʧ������ҺŨ��ƫС��
E��δϴ���ܽ�NaOH���ձ����ᵼ�����ʵ���ʧ������ҺŨ��ƫС��
F������ʱ���ӿ̶��ߣ��ᵼ����Һ���ƫ����Ũ��ƫС��
G��ֻҪ�����ʱ��Һ����̶������м��ɣ�����ˮ�����Ⱦ��еĻ��Ǻ�������ģ������ʵ����ʵ�����������Һ���������Ӱ�죬���Ũ����Ӱ�죻
H�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶����������������������������ڿ̶������ϵ���Һ�������䣬������̶�����ƽ����Ӱ����Һ��������ټ�ˮ���̶��ߵ���Ũ��ƫС��
��Ũ��ƫ����У�AC��ƫС���У�BDEFH����Ӱ����У�G��
�ʴ�Ϊ��AC��BDEFH��G��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ������漰����������ѡ���㡢�����������⣬��Ŀ�ѶȲ���ע���c=$\frac{n}{V}$��������ԭ����


 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 7�� | B�� | 8�� | C�� | 9�� | D�� | 10�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������������ѧ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ����
A��ͬ��ͬѹ�£�H2��g��+Cl2��g��=2HCl��g���ڹ��պ͵�ȼ�������¡�H����ͬ
B����ѧ��Ӧ�е������仯������Ϊ�����仯
C���κη��ȷ�Ӧ�ڳ����¶��ܷ���
D����ѧ��Ӧ�ķ�Ӧ�ȿ�ͨ����Ӧ��ļ���֮�ͼ�ȥ������ļ���֮�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
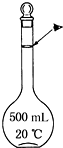 ʵ��������500mL 0.5mol•L-1��NaCl��Һ�������²������裺
ʵ��������500mL 0.5mol•L-1��NaCl��Һ�������²������裺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ս̰桶��ѧ1�������̽����������100mL 0.100mol•L-1 Na2CO3��Һ��
�ս̰桶��ѧ1�������̽����������100mL 0.100mol•L-1 Na2CO3��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������и�һ��9�µ��л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��������Һ��1 L 0.1 mol��L��1 NaCl��Һ��ϣ�������Һc(Cl��)������
A��50 mL 1 mol��L��1 NaCl��Һ B��20 mL 2 mol��L��1 A lCl3��Һ
lCl3��Һ
C��30 mL 1 mol��L��1 MgCl2��Һ D��100 mL 3 mol��L��1 KClO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����NaOH���� | B�� | ��������ƾ��� | C�� | �������� | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH�����
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH�����| ʵ���� | HA���ʵ���Ũ�� ��mol•L-1�� | NaOH���ʵ���Ũ�� ��mol•L-1�� | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=9 |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com