
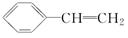 £®
£® £¬D
£¬D £¬E
£¬E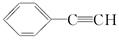 £¬H
£¬H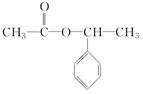 £®
£® +NaOH$”ś_{”÷}^{Ė®}$
+NaOH$”ś_{”÷}^{Ė®}$ +NaBr£®Š“³ö¢ß·“Ó¦µÄ»Æѧ·½³ĢŹ½
+NaBr£®Š“³ö¢ß·“Ó¦µÄ»Æѧ·½³ĢŹ½ +CH3COOH$”ś_{”÷}^{ÅØĮņĖį}$
+CH3COOH$”ś_{”÷}^{ÅØĮņĖį}$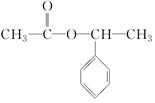 +H2O£®
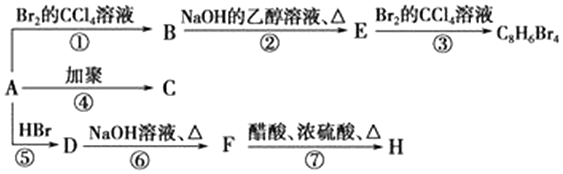
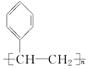
+H2O£® ·ÖĪö 1 molĢžAĶźČ«Č¼ÉÕµĆ8 mol CO2ŗĶ4 mol H2O£¬ŌņAµÄ·Ö×ÓŹ½ĪŖC8H8£¬¢Ł·¢ÉśµÄŹĒ¼Ó³É·“Ó¦£¬·“Ó¦¢ŚŹĒ“Ó·Ö×ÓBÖŠĻūČ„2·Ö×ÓHBr£¬¢ŪŹĒŌŚEÖŠ¼ÓČė2·Ö×ÓBr2£®ŌņAĪŖ£ŗ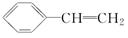 £¬BĪŖ£ŗ
£¬BĪŖ£ŗ £¬EĪŖ£ŗ
£¬EĪŖ£ŗ £¬CĪŖ£ŗ
£¬CĪŖ£ŗ £¬½įŗĻ²»¶Ō³ĘĻ©ĢžÓėHBr¼Ó³É·“Ó¦æÉDĪŖ£ŗ
£¬½įŗĻ²»¶Ō³ĘĻ©ĢžÓėHBr¼Ó³É·“Ó¦æÉDĪŖ£ŗ £¬FĪŖ£ŗ
£¬FĪŖ£ŗ £¬HĪŖ£ŗ
£¬HĪŖ£ŗ £®
£®
½ā“š ½ā£ŗ1 molĢžAĶźČ«Č¼ÉÕµĆ8 mol CO2ŗĶ4 mol H2O£¬ŌņAµÄ·Ö×ÓŹ½ĪŖC8H8£¬¢Ł·¢ÉśµÄŹĒ¼Ó³É·“Ó¦£¬·“Ó¦¢ŚŹĒ“Ó·Ö×ÓBÖŠĻūČ„2·Ö×ÓHBr£¬¢ŪŹĒŌŚEÖŠ¼ÓČė2·Ö×ÓBr2£®ŌņAĪŖ£ŗ £¬BĪŖ£ŗ
£¬BĪŖ£ŗ £¬EĪŖ£ŗ
£¬EĪŖ£ŗ £¬CĪŖ£ŗ
£¬CĪŖ£ŗ £¬½įŗĻ²»¶Ō³ĘĻ©ĢžÓėHBr¼Ó³É·“Ó¦æÉDĪŖ£ŗ
£¬½įŗĻ²»¶Ō³ĘĻ©ĢžÓėHBr¼Ó³É·“Ó¦æÉDĪŖ£ŗ £¬FĪŖ£ŗ
£¬FĪŖ£ŗ £¬HĪŖ£ŗ
£¬HĪŖ£ŗ £®
£®
£Ø1£©ÓÉÉĻŹö·ÖĪö£¬æÉÖŖAµÄ»ÆѧŹ½£ŗC8H8£¬AµÄ½į¹¹¼ņŹ½ĪŖ £¬
£¬
¹Ź“š°øĪŖ£ŗC8H8£» £»
£»
£Ø2£©ÉĻŹö·“Ó¦ÖŠ£¬¢ŁŹĒ¼Ó³É·“Ó¦£¬¢ßŹĒõ„»Æ·“Ó¦»ņČ”“ś·“Ó¦£¬
¹Ź“š°øĪŖ£ŗ¼Ó³É£»õ„»Æ»ņČ”“ś£»
£Ø3£©ÓÉÉĻŹö·ÖĪö£¬æÉÖŖ£¬CĪŖ£ŗ £¬DĪŖ£ŗ
£¬DĪŖ£ŗ £¬EĪŖ£ŗ
£¬EĪŖ£ŗ £¬HĪŖ£ŗ
£¬HĪŖ£ŗ £®
£®
¹Ź“š°øĪŖ£ŗ £»
£» £»
£» £»
£» £»
£»
£Ø4£©¢Ž·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ +NaOH$”ś_{”÷}^{Ė®}$
+NaOH$”ś_{”÷}^{Ė®}$ +NaBr£¬¢ß·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
+NaBr£¬¢ß·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ +CH3COOH$”ś_{”÷}^{ÅØĮņĖį}$
+CH3COOH$”ś_{”÷}^{ÅØĮņĖį}$ +H2O£¬
+H2O£¬
¹Ź“š°øĪŖ£ŗ +NaOH$”ś_{”÷}^{Ė®}$
+NaOH$”ś_{”÷}^{Ė®}$ +NaBr£»
+NaBr£» +CH3COOH$”ś_{”÷}^{ÅØĮņĖį}$
+CH3COOH$”ś_{”÷}^{ÅØĮņĖį}$ +H2O£®
+H2O£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄĶʶĻ£¬³ä·ÖĄūÓĆAÓė¢ŪµÄ²śĪļ·Ö×ÓŹ½£¬½įŗĻ·“Ó¦Ģõ¼ž½ųŠŠĶʶĻ£¬ŹģĮ·ÕĘĪÕ¹ŁÄÜĶŵÄŅżČėÓėĻū³ż£¬ŹĒ¶ŌÓŠ»ś»Æѧ»ł“”µÄ×ŪŗĻ漲飮



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | øɱłÕō·¢ŅŖĪüŹÕ“óĮæµÄČČ£¬Õā²»ŹĒ»Æѧ·“Ó¦ÖŠµÄĪüČČĻÖĻó | |
| B£® | ¾Ę¾«³£±»ÓĆ×÷¾Ę¾«µĘŗĶÄŚČ¼»śÖŠµÄČ¼ĮĻ£¬ĖµĆ÷¾Ę¾«Č¼ÉÕŹĒ·ÅČČ·“Ó¦ | |
| C£® | ľĢæ³£ĪĀĻĀ²»Č¼ÉÕ£¬¼ÓČČ²ÅÄÜČ¼ÉÕ£¬ĖµĆ÷ľĢæČ¼ÉÕŹĒĪüČČ·“Ó¦ | |
| D£® | ČĖĆĒÓĆĒāŃõŃęŗø½Ó½šŹō£¬Ö÷ŅŖŹĒĄūÓĆĮĖĒāĘųŗĶŃõĘų»ÆŗĻŹ±Ėł·Å³öµÄÄÜĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·“Ó¦¢ŁŹ¹ÓĆ“ß»Æ¼Į£¬ÄܽµµĶøĆ·“Ó¦µÄ»ī»ÆÄÜ | |
| B£® | ·“Ó¦¢ŚĪŖĪüČČ·“Ó¦ | |
| C£® | ·“Ó¦¢Ū½«»ÆѧÄÜ×Ŗ»ÆĪŖ¹āÄÜ | |
| D£® | ·“Ó¦CO£Øg£©+H2O£Øg£©ØTCO2£Øg£©+H2£Øg£©£»”÷H=-82.4 kJ•mol-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÅØ°±Ė® | B£® | NaOHČÜŅŗ | C£® | ŃĪĖį | D£® | CO2ŗĶĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na”¢Mg”¢AlµÄ½šŹōŠŌŅĄ“Ī¼õČõ | B£® | H2S”¢H2O”¢HFµÄĪČ¶ØŠŌŅĄ“Ī¼õČõ | ||
| C£® | Cl-”¢Br-”¢I- »¹ŌŠŌŅĄ“ĪŌöĒæ | D£® | Na”¢K”¢RbµÄŌ×Ó°ė¾¶ŅĄ“ĪŌö“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
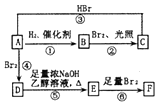 Ļ©ĢžAŌŚŅ»¶ØĢõ¼žĻĀæÉŅŌ°“ĻĀ¶ųµÄĮ÷³Ģ½ųŠŠ·“Ó¦£®
Ļ©ĢžAŌŚŅ»¶ØĢõ¼žĻĀæÉŅŌ°“ĻĀ¶ųµÄĮ÷³Ģ½ųŠŠ·“Ó¦£®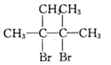
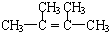 £®
£®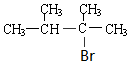 £®
£®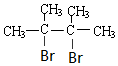 +2NaOH$”ś_{”÷}^{ŅŅ“¼}$
+2NaOH$”ś_{”÷}^{ŅŅ“¼}$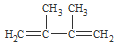 +2NaBr+2H2O£®
+2NaBr+2H2O£®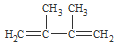 +2Br2”ś
+2Br2”ś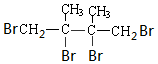 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
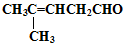 ¼ģŃé·Ö×ÓÖŠČ©»łµÄĖłÓƵďŌ¼ĮŹĒŅų°±ČÜŅŗ£Ø»ņŠĀÖʱøĒāŃõ»ÆĶ×ĒŅŗ£©£¬»Æѧ·½³ĢŹ½ĪŖ
¼ģŃé·Ö×ÓÖŠČ©»łµÄĖłÓƵďŌ¼ĮŹĒŅų°±ČÜŅŗ£Ø»ņŠĀÖʱøĒāŃõ»ÆĶ×ĒŅŗ£©£¬»Æѧ·½³ĢŹ½ĪŖ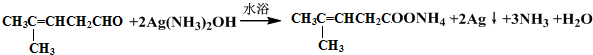 »ņ
»ņ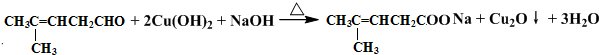 £»
£»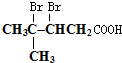 £»
£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻņijČÜŅŗÖŠ¼ÓČėĻ”ĻõĖįĖį»Æ£¬ŌŁµĪČėBaCl2ČÜŅŗ£¬²śÉś°×É«³Įµķ£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠSO42- | |
| B£® | ĻņijČÜŅŗÖŠ¼ÓČėAgNO3ČÜŅŗ£¬²śÉś°×É«³Įµķ£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠCl-? | |
| C£® | ÓĆ¹ā½ąµÄ²¬ĖæÕŗČ”Ä³ĪŽÉ«ČÜŅŗ£¬ŌŚ¾Ę¾«µĘĶāŃęĄļ×ĘÉÕŹ±¹Ū²ģµ½»ĘÉ«»šŃę£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠNa+ | |
| D£® | ĻņijČÜŅŗÖŠ¼ÓČėĢ¼ĖįÄĘČÜŅŗ£¬²śÉś°×É«³Įµķ£¬ŌŁµĪČėĻ”ŃĪĖį£¬³ĮµķČܽā£¬ŌņŌČÜŅŗÖŠŅ»¶ØÓŠCa2+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ź¢×°Ī“ÖŖŅŗµÄ׶ŠĪĘæÓĆÕōĮóĖ®Ļ“¹ż£¬Ī“ÓĆĪ“ÖŖŅŗČóĻ“ | |
| B£® | µĪ¶ØÖÕµć¶ĮŹżŹ±£¬ø©ŹÓµĪ¶Ø¹ÜµÄæĢ¶Č£¬ĘäĖū²Ł×÷ÕżČ· | |
| C£® | µĪ¶Øµ½ÖÕµć¶ĮŹżŹ±£¬·¢ĻÖµĪ¶Ø¹Ü¼ā×ģÄŚÓŠĘųÅŻ | |
| D£® | ÅäÖʱź×¼ČÜŅŗµÄNaOHÖŠ»ģÓŠKOHŌÓÖŹ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com