
��14�֣���ͭ�����ɷ�ΪCu2S��ͨ�����Ŀ�����ұ������ͭ������һϵ�з�Ӧ�ɵõ�B��D��E��GΪש��ɫ������
��ش��������⣺
��1����ͭ��Cu2S��ͨ�����Ŀ���ұ������ͭ�Ļ�ѧ����ʽ__________________________
______����������Ϊ____________________��
��2��E��Ũ��Һ��Cu������Ӧ�ڵĻ�ѧ����ʽ�� ��
��3�����õ����ᴿ�֣��ڸõ�ⷴӦ������������ ���������Һ�� ��
��4����Ȼ���е��������������FeS2��������������ʱ�Ỻ���������з�Ӧ������ͭ�Է�Ӧ��14CuSO4+ 5FeS2 +12H2O==7Cu2S+5FeSO4+12H2SO4
����������ͱ���ԭ�����������Ϊ_________________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣� ��ͭ�����ɷ�ΪCu2S��ͨ�����Ŀ�����ұ������ͭ������һϵ�з�Ӧ�ɵõ�B��D��E��GΪש��ɫ������
��ش��������⣺
��1����ͭ��Cu2S��ͨ�����Ŀ���ұ������ͭ�Ļ�ѧ����ʽ__________________________
______����������Ϊ____________________��
��2��E��Ũ��Һ��Cu������Ӧ�ڵĻ�ѧ����ʽ�� ��
��3�����õ����ᴿ�֣��ڸõ�ⷴӦ������������ ���������Һ�� ��
��4����Ȼ���е��������������FeS2��������������ʱ�Ỻ���������з�Ӧ������ͭ�Է�Ӧ��14CuSO4+ 5FeS2 +12H2O==7Cu2S+5FeSO4+12H2SO4
����������ͱ���ԭ�����������Ϊ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ���ϡ��㱱����ѧУ����12��������ѧ�Ծ� ���ͣ������
��8�֣���ͭ�����ɷ�ΪCu2S��ͨ�����Ŀ�����ұ������ͭ������һϵ�з�Ӧ�ɵõ�B��D��E��GΪש��ɫ������
��ش��������⣺
��1����ͭ��Cu2S��ͨ �����Ŀ���ұ������ͭ�Ļ�ѧ����ʽ__________________________
�����Ŀ���ұ������ͭ�Ļ�ѧ����ʽ__________________________
______����������Ϊ____________________��
��2��E��Ũ��Һ��Cu������Ӧ�ڵĻ�ѧ����ʽ�� ��
��3�����õ����ᴿ�֣��ڸõ�ⷴӦ������������ ���������Һ�� ��
��4����Ȼ���е��������������FeS2��������������ʱ�Ỻ���������з�Ӧ������ͭ�Է�Ӧ��14CuSO4+ 5FeS2 +12H2O==7Cu2S+5FeSO 4+12H2SO4
4+12H2SO4
����������ͱ���ԭ�����������Ϊ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����12��������ѧ�Ծ� ���ͣ������
��8�֣���ͭ�����ɷ�ΪCu2S��ͨ�����Ŀ�����ұ������ͭ������һϵ�з�Ӧ�ɵõ�B��D��E��GΪש��ɫ������
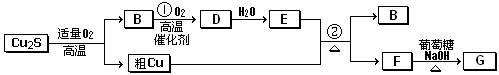
��ش��������⣺
��1����ͭ��Cu2S��ͨ�����Ŀ���ұ������ͭ�Ļ�ѧ����ʽ__________________________
______����������Ϊ____________________��
��2��E��Ũ��Һ��Cu������Ӧ�ڵĻ�ѧ����ʽ�� ��
��3�����õ����ᴿ�֣��ڸõ�ⷴӦ������������ ���������Һ�� ��
��4����Ȼ���е��������������FeS2��������������ʱ�Ỻ���������з�Ӧ������ͭ�Է�Ӧ��14CuSO4+ 5FeS2 +12H2O==7Cu2S+5FeSO4+12H2SO4
����������ͱ���ԭ�����������Ϊ_________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com