
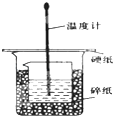
 ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶ȣ�t2��/�� | �²� ��t2-t1��/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
���� ��1���������ȼƵĹ������жϸ�װ�õ�ȱ���������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��2���²t2-t1��Ӧȡ����ʵ���ƽ��ֵ6.7���������к��ȣ������к��ȵĸ���д�����кͷ�Ӧ���Ȼ�ѧ����ʽ��
��3���ô���������ᣬ�������Ҫ������������õ�����ƫС��
��4��û����ˮϴ���¶ȼ��ϵ�������Һ�����²ⶨNaOH��Һ�¶�ƫ�ߣ��¶Ȳ�ƫС����õ�����ƫС��
��� �⣺��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β�����������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮����������ĭ���ϵ������Ǽ���ʵ������е�������ʧ��
�ʴ�Ϊ�����β�������������¸��ȣ���ֹ����ɢʧ��
��2���������β������ݶ�����Ч�ģ������²��ƽ��ֵΪ��$\frac{6.6��+6.7��+6.8��}{3}$=6.7�棬��H=-$\frac{cm��t}{n��H2O��}$=-$\frac{4.18��1{0}^{-3}KJ/��g•�棩����50+50��mL��1.00g/mL��6.7��}{0.05L��1.0mol/L}$=-56.0kJ/mol�������к��ȵĸ����֪���Ȼ�ѧ����ʽΪ��H+��aq��+OH-��aq��=H2O��l����H=-56.0kJ/mol��
�ʴ�Ϊ��H+��aq��+OH-��aq��=H2O��l����H=-56.0 kJ/mol��
��3���ô���������ᣬ�������Ҫ������������÷ų�������ƫС����Ϊ�к��ȡ�HΪ��ֵ�������к��ȡ�Hƫ�ʴ�Ϊ��ƫ��
��4��û����ˮϴ���¶ȼ��ϵ�������Һ�����²ⶨNaOH��Һ�¶�ƫ�ߣ��¶Ȳ�ƫС����õ�����ƫС����Ϊ�к��ȡ�HΪ��ֵ�������к��ȡ�Hƫ�ʴ�Ϊ��ƫ��
���� ���⿼�����к��ȵIJⶨ����Ŀ�Ѷ��еȣ�ע�������к��ȵIJⶨ��������ȷ�к��Ȳⶨ�б��뾡������������ɢʧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
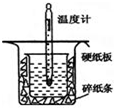 ��1���� 0.50mol•L-1������50mL 0.55mol•L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺
��1���� 0.50mol•L-1������50mL 0.55mol•L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺| ��ѧ�� | H-H | N-H | N��N |
| ����kJ•mol-1 | 436 | 391 | 945 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����д����Ӧ��Ļ�ѧ����ʽ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
����д����Ӧ��Ļ�ѧ����ʽ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״����1molSO3�����Ϊ22.4L | B�� | ��״����CO2���ܶȱ�SO2�� | ||
| C�� | 1molSO42-������Ϊ94g | D�� | 1molOH-����10mol���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ͼ���������У�a��b��c��d��Ϊʯī�缫�����������й���0.02mol����ͨ����������������ȷ���ǣ�������
��ͼ���������У�a��b��c��d��Ϊʯī�缫�����������й���0.02mol����ͨ����������������ȷ���ǣ�������| A�� | ���ձ���a������������ͭ0.64g | |
| B�� | ���ձ���b���ϵ缫��Ӧʽ4OH--4e-�T2H2O+O2�� | |
| C�� | ���ձ��е����̪��Һ��d�������ȱ�� | |
| D�� | �ձ���c���ϵ缫��ӦʽΪ4H++4e-�T2H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
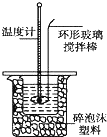 ijʵ��С����0.50mol•L-1NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol•L-1NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ� ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��50mL0.50mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����п��ܺ�����Ԫ�� | B�� | ������һ��������Ԫ�� | ||
| C�� | ����һ��������Ԫ�غ���Ԫ�� | D�� | ��ʽ���к�����Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com