
��֪��������ͨ����������S8(б����)����ʽ���ڣ���������״̬ʱ������S2��S4��S6��S8�ȶ���ͬ�������壬����S4��S6��S8�������ƵĽṹ�ص㣬��ṹ����ͼ��ʾ��
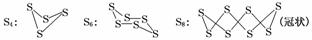
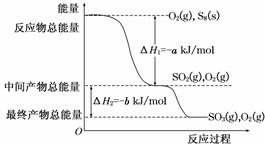
��һ�������£�S8(s)��O2(g)������Ӧ����ת��ΪSO2(g)��SO3(g)����Ӧ���̺�������ϵ������ͼ��ʾ(ͼ�еĦ�H��ʾ����1 mol���������)��
(1)д����ʾS8ȼ���ȵ��Ȼ�ѧ����ʽ_________________________________
____________��
(2)д��SO3�ֽ�����SO2��O2���Ȼ�ѧ����ʽ______________________________
________________________________________________________________________��
(3)��ѧ�Ϲ涨�����γ�1 mol��ѧ�����ջ�ų���������Ϊ�û�ѧ���ļ��ܣ���λkJ��mol������֪�������ļ���Ϊd kJ��mol��1���������ļ���Ϊe kJ��mol��1����S8������������ļ���Ϊ____________________________________��
������(1)ȼ����ָ����1 molȼ����ȫȼ�������ȶ�������ʱ�ų�����������˸��ݶ�����д����ѧ����ʽ��S8(s)��8O2(g) ,��8SO2(g)������������и������ʱ���ָ����1 mol��������ݣ���˸÷�Ӧ���Ȼ�ѧ����ʽΪS8(s)��8O2(g)===8SO2(g)����H����8a kJ��mol��1��
(2)�ɸ�������ͼʾֱ��д���Ȼ�ѧ����ʽ��
(3)��S8������������ļ���Ϊx kJ��mol��1�����ڷ�Ӧ�ȵ��ڷ�Ӧ��ļ���֮�ͼ�ȥ������ļ���֮�ͣ���Ϣٿɵ�8x��8e��16d����8a�����x��2d��a��e��
�𰸣�(1)S8(s)��8O2(g)===8SO2(g)����H����8a kJ��mol��1
(2)2SO3(g)===2SO2(g)��O2(g)����H����2b kJ��mol��1
(3)(2d��a��e) kJ��mol��1



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ������4��̼ԭ�ӽ�ϳɵ�6���л���(��ԭ��û�л���)��
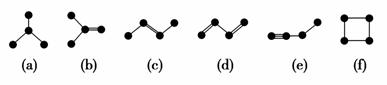
(1)�л���(a)��һ��ͬ���칹�壬��д����ṹ��ʽ________��
(2)�����л�������(c)��Ϊͬ���칹�����________(�����)��
(3)��дһ����(e)��Ϊͬϵ����л���Ľṹ��ʽ________��
(4)(a)(b)(c)(d)(e)���������У�4��̼ԭ��һ������ͬһƽ�����________(�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij������ˮ��Һ�����ܺ������������е������֣�K����Al3����Fe3����Mg2����Ba2����NH ��Cl����CO
��Cl����CO ��SO
��SO ���ֱַ�ȡ100 mL�����ȷ���Һ��������ʵ�飺
���ֱַ�ȡ100 mL�����ȷ���Һ��������ʵ�飺
�ٵ�һ�ݼӹ���NaOH��Һ����ȣ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס�
�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02 g���塣
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65 g���塣
(1)һ�������ڵ�������________(�����ӷ��ţ���ͬ)��
(2)�ɢٿ�֪��������Ϊ________��Ũ��________���ɢڿ�֪��������Ϊ________��Ũ��________��
�ɢۿ�֪��������Ϊ________��Ũ��________��
(3)K���Ƿ���ڣ�________(��ǡ���)��������___________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȼ�ϵ������Ч�����Դ�����ʣ����й㷺��Ӧ��ǰ�����������ʾ�������ȼ�ϵ�ص�ȼ�ϣ������������(����)
A���״������������������� ��B����Ȼ��
C��Һ��ʯ���� D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��NH3��H2O(aq)��H2SO4(aq)��Ӧ����1 mol���εĦ�H����24.2 kJ��mol��1��ǿ�ᡢǿ���ϡ��Һ��Ӧ���к���Ϊ��H����57.3 kJ��mol��1����NH3��H2O��ˮ��Һ�е���Ħ�H����(����)
A����69.4 kJ��mol��1 B����45.2 kJ��mol��1
C����69.4 kJ��mol��1 D����45.2 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д�����л���������ƻ�ṹ��ʽ��
(1)ij���Ľṹ��ʽΪ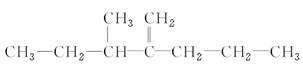 ����������Ϊ_______________________________________________________________��
����������Ϊ_______________________________________________________________��
(2)ij���Ľṹ��ʽΪ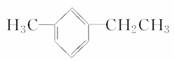 ��������Ϊ______________
��������Ϊ______________
__________________________________________________________��
(3)2,5����2,4����ϩ�Ľṹ��ʽΪ_______________________________
_________________________________________��
(4)ij���Ľṹ��ʽΪ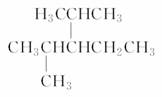 ��������Ϊ_______________
��������Ϊ_______________
________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���п���������SO2��CO2������ǣ� ��
�ٳ����ʯ��ˮ�����������ˮ�����Ը�����آ��Ȼ�����Ʒ����Һ
| A���٢ܢݢ� | B���ڢۢܢ� | C���٢ڢۢ� | D���ڢۢܢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com