ĄŸŽđ°žĄż
·ÖÎöŁșŁš1Ł©ąÙŽÓÎïÖÊ”ÄŚéłÉșÍĐÔÖʜǶȷÖÎöŁŹČÏËżÊôÓÚ”°°ŚÖÊŁŹÌÇÀàČ»Ò»¶šÓĐÌđζŁŹÀęÈç”í·ÛșÍÏËÎŹËŰÊôÓÚÌÇŁŹ”«Ă»ÓĐÌđζŁ»ÁòËáï§ÈÜÒșŒÓÈ딜”°°ŚÖÊÈÜÒșÖĐŁŹÄÜÊč”°°ŚÖÊ·ąÉúŃÎÎöŁŹÒÒËáÇŠÈÜÒșŒÓÈ딜”°°ŚÖÊÈÜÒșÖĐŁŹÄÜÊč”°°ŚÖÊ·ąÉú±äĐÔŁ»
ąÚÆÏÌŃÌÇÎȘ¶àôÇ»ùÈ©ÊÇ»čÔĐÔ”„ÌÇŁŹÒÔŽËÊéĐŽ»ŻŃ§·œłÌÊœŁ»
Łš2Ł©žùŸĘÓĐ»úÎï”ÄœáččŒòÊœÀŽčÛČìșŹÓĐ”ÄčÙÄÜÍĆŁŹČąÊéĐŽĂûłÆŁ»-COOHÓë-OH·ąÉúő„»Ż·ŽÓŠÉúłÉő„Ł»ÉúłÉÂíÀŽËᣏÂíÀŽËáÄÜÊčäćËźÍÊÉ«ŁŹË”ĂśșŹÓĐ̌̌˫ŒüŁ»
Łš3Ł©ąÙÒșÌćŒÓÈÈÊčÒŚ±©·ĐŁŹÓŠŒÓÈëËéŽÉÆŹŁ»
ąÚ»ŻŃ§·ŽÓŠŽï”œÆœșâʱŁŹŐęÄæ·ŽÓŠËÙÂÊÏà”ÈŁŹžśÎïÖÊ”ÄĆš¶ÈČ»±äŁŹÓÉŽËŃÜÉú”ÄÒ»Đ©ÎïÀíÁżÒČČ»±äŁ»
ąÛŽÖČúÆ·ÒÒËáÒÒő„ÖĐșŹÓĐÒÒËáÓëÒÒŽŒŁŹÓñ„șÍ”ÄÌŒËáÄÆÈÜÒș·ŽÓŠ”ôÒÒËᣏÈÜœâÒÒŽŒŁŹÍŹÊ±œ””ÍÒÒËáÒÒő„”ÄÈÜœâ¶ÈŁŹ±ăÓÚÈÜÒș·ÖČ㣏ÒÒËáÒÒő„ĂܶȱÈ˟ХŁŹÒÒËáÒÒő„ÔÚÉÏČăŁŹÈ»șóÀûÓĂ·ÖÒșŁŹÒÒËáÒÒő„ŒÓÈëÎȚËźÌŒËáÄÆÎüÊŐÆäÖĐ”ÄËźŁŹżÉ”ĂÒÒËáÒÒő„Ł»ÈÜÒșÖĐșŹÓĐÒÒŽŒĄąÌŒËáÄÆĄąÒÒËáÄÆŁŹœűĐĐŐôÁóżÉÒÔÊŐŒŻÒÒŽŒŁŹÏòŐôłöÒÒŽŒ”ÄÈÜÒșÖĐŒÓÈëÁòËᣏżÉÒԔÔœÒÒËᣏÔÙœűĐĐŐôÁóżÉÒÔÊŐŒŻÒÒË᣻
ąÜÁòËá”ÄËáĐÔÇżÓÚÒÒËᣟ
œâŽđŁșœâŁșŁš1Ł©ąÙAŁźĂȚĄąÂéĄąÄŸČÄ”ÄÖśÒȘłÉ·ÖÎȘÏËÎŹËŰŁŹ”«ČÏËż”ÄÖśÒȘłÉ·Ö”°°ŚÖÊŁŹčÊAŽíÎóŁ»
BŁź»ù±ŸÓȘŃűÎïÖÊÓĐÌÇÀàĄąÓÍÖŹĄą”°°ŚÖÊ”ÈŁŹÆäÖĐÓÍÖŹČúÉú”ÄÄÜÁżŚîžßŁŹčÊBŐęÈ·Ł»
CŁź”°°ŚÖÊÊÇ°±»ùËá”ÄËőŸÛČúÎïŁŹËźœâŚîÖŐÉúłÉ°±»ùËᣏčÊCŐęÈ·Ł»
DŁźÌÇÀàČ»Ò»¶šÓĐÌđζŁŹÀęÈç”í·ÛșÍÏËÎŹËŰÊôÓÚÌÇŁŹ”«Ă»ÓĐÌđζŁŹčÊDŽíÎóŁ»
EŁź”í·ÛĄąÏËÎŹËŰĄą”°°ŚÖʶŒÊÇÌìÈ»žß·ÖŚÓ»ŻșÏÎïŁŹÏà¶Ô·ÖŚÓÖÊÁżœÏŽóŁŹčÊEŐęÈ·Ł»
FŁźÁòËáï§ÊôÓÚ·ÇÖŰœđÊôŃÎŁŹËùÒÔÁòËáï§ÈÜÒșŒÓÈ딜”°°ŚÖÊÈÜÒșÖĐŁŹÄÜÊč”°°ŚÖÊ·ąÉúŃÎÎöŁŹÒÒËáÇŠÊôÓÚÖŰœđÊôŃÎŁŹËùÒÔÒÒËáÇŠÈÜÒșŒÓÈ딜”°°ŚÖÊÈÜÒșÖĐŁŹÄÜÊč”°°ŚÖÊ·ąÉú±äĐÔŁŹ”°°ŚÖʶŒÄÜŽÓÈÜÒșÖĐÎöłöŁŹčÊFŐęÈ·ŁŹ
čÊŽđ°žÎȘŁșBCEFŁ»
ąÚÆÏÌŃÌÇÎȘ»čÔĐÔ”„ÌÇŁŹÎȘ¶àôÇ»ùÈ©ŁŹÆÏÌŃÌÇÔÚËźÔĄŒÓÈÈÌőŒțÏ·ąÉúÒűŸ”·ŽÓŠŁŹ·ŽÓŠ”Ä·œłÌÊœÎȘCH
2OHŁšCHOHŁ©
4CHO+2AgŁšNH
3Ł©
2OH

2AgĄę+CH
2OHŁšCHOHŁ©
4COONH
4+3NH
3+H
2OŁŹ
čÊŽđ°žÎȘŁșCH
2OHŁšCHOHŁ©
4CHO+2AgŁšNH
3Ł©
2OH

2AgĄę+CH
2OHŁšCHOHŁ©
4COONH
4+3NH
3+H
2OŁ»
Łš2Ł©žùŸĘÓĐ»úÎï”ÄœáččŒòÊœżÉÖȘžĂÓĐ»úÎïșŹÓĐșŹÓĐ-COOHĄą-OHÁœÖÖčÙÄÜÍĆŁŹĂûłÆÎȘôÈ»ùșÍôÇ»ùŁ»ôÈ»ùșÍôÇ»ùŒäÄÜ·ąÉúő„»Ż·ŽÓŠŁ»Æ»čûËá·ąÉú·ÖŚÓÄÚÍŃËź·ŽÓŠ”Ä·œłÌÊœÎȘHOOCCHŁšOHŁ©CH
2COOH
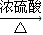
HOOCCH=CHCOOH+H
2OŁŹ
čÊŽđ°žÎȘŁșôÇ»ùŁ»ôÈ»ùŁ»ő„»ŻŁ»HOOC-CH=CH-COOHŁ»
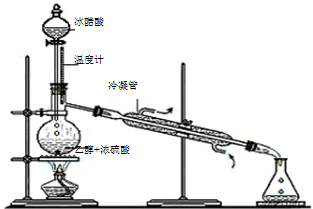
Łš3Ł©ąÙÒșÌćŒÓÈÈÊčÒŚ±©·ĐŁŹÓŠŒÓÈëËéŽÉÆŹŁŹčÊŽđ°žÎȘŁș·ÀÖčÒșÌćÊÜÈȱ©·ĐŁ»
ąÚÒŃÖȘżÉÄæ·ŽÓŠCH
3CH
2OH+CH
3COOH

CH
3COOCH
2CH
3+H
2OŁŹ
AŁź»ŻŃ§·ŽÓŠËÙÂÊÖź±È”ÈÓÚ»ŻŃ§ŒÆÁżÊęÖź±ÈŁŹÎȚÂÛÊÇ·ńŽï”œÆœș⣏ÉúłÉ1molÒÒËáÒÒő„ŁŹÍŹÊ±ÉúłÉ1molËźŁŹčÊAŽíÎóŁ»
BŁź”„λʱŒäÀïŁŹÉúłÉ1molÒÒËáÒÒő„ŁŹÍŹÊ±ÉúłÉ1molÒÒËᣏ˔ÜŐęÄæ·ŽÓŠËÙÂÊÏà”ÈŁŹčÊBŐęÈ·Ł»
CŁź»ŻŃ§·ŽÓŠËÙÂÊÖź±È”ÈÓÚ»ŻŃ§ŒÆÁżÊęÖź±ÈŁŹÎȚÂÛÊÇ·ńŽï”œÆœș⣏”„λʱŒäÀïŁŹÏûșÄ1molÒÒŽŒŁŹÍŹÊ±ÏûșÄ1molÒÒËᣏčÊCŽíÎóŁ»
DŁźŐę·ŽÓŠ”ÄËÙÂÊÓëÄæ·ŽÓŠ”ÄËÙÂÊÏà”ÈŁŹŽï”œÆœșâŚŽÌŹŁŹčÊDŐęÈ·Ł»
EŁź»ìșÏÎïÖĐžśÎïÖÊ”ÄĆš¶ÈČ»ÔÙ±ä»ŻŁŹË”ĂśŽï”œÆœșâŚŽÌŹŁŹčÊEŐęÈ·Łź
čÊŽđ°žÎȘŁșBDEŁź
ąÛŽÖČúÆ·ÒÒËáÒÒő„ÖĐșŹÓĐÒÒËáÓëÒÒŽŒŁŹÓñ„șÍ”ÄÌŒËáÄÆÈÜÒș·ŽÓŠ”ôÒÒËᣏÈÜœâÒÒŽŒŁŹÍŹÊ±œ””ÍÒÒËáÒÒő„”ÄÈÜœâ¶ÈŁŹ±ăÓÚÈÜÒș·ÖČ㣏ÒÒËáÒÒő„ĂܶȱÈ˟ХŁŹÒÒËáÒÒő„ÔÚÉÏČăŁŹÈ»șóÀûÓĂ·ÖÒșŁŹÒÒËáÒÒő„ŒÓÈëÎȚËźÌŒËáÄÆÎüÊŐÆäÖĐ”ÄËźŁŹżÉ”ĂÒÒËáÒÒő„Ł»ÈÜÒșÖĐșŹÓĐÒÒŽŒĄąÌŒËáÄÆĄąÒÒËáÄÆŁŹœűĐĐŐôÁóżÉÒÔÊŐŒŻÒÒŽŒŁŹÏòŐôłöÒÒŽŒ”ÄÈÜÒșÖĐŒÓÈëÁòËᣏżÉÒԔÔœÒÒËᣏÔÙœűĐĐŐôÁóżÉÒÔÊŐŒŻÒÒË᣻
čÊŽđ°žÎȘŁș±„șÍNa
2CO
3ÈÜÒșŁ»·ÖÒșŁ»ŐôÁ󣻣šĆšŁ©ÁòË᣻
ąÜÒÒËáÄÆÎȘÈőËáŃÎŁŹÁòËáÎȘÇżËᣏžùŸĘÇżËáÖÆÈőËᣏ2CH
3COONa+H
2SO
4Ąú2CH
3COOH+Na
2SO
4ŁŹčÊŽđ°žÎȘŁș2CH
3COONa+H
2SO
4Ąú2CH
3COOH+Na
2SO
4Łź
”ăÆÀŁș±ŸÌâżŒČéœÏÎȘŚÛșÏŁŹÉ挰ÓĐ»úÎï”ÄŚéłÉĄąœáččșÍĐÔÖÊÒÔŒ°ÒÒËáÒÒő„”ÄÖƱžŁŹŚąÒâÒÒËáÒÒő„·ŽÓŠ”ÄżÉÄæĐÔÌ۔㣏ŚąÒâĆжÏÆœșâŚŽÌŹ”Ä·œ·šŁŹÎȘÒŚŽí”㣏ÌâÄżÄѶÈÖĐ”ÈŁź

 2AgĄę+CH2OHŁšCHOHŁ©4COONH4+3NH3+H2OŁŹ
2AgĄę+CH2OHŁšCHOHŁ©4COONH4+3NH3+H2OŁŹ 2AgĄę+CH2OHŁšCHOHŁ©4COONH4+3NH3+H2OŁ»
2AgĄę+CH2OHŁšCHOHŁ©4COONH4+3NH3+H2OŁ» HOOCCH=CHCOOH+H2OŁŹ
HOOCCH=CHCOOH+H2OŁŹ CH3COOCH2CH3+H2OŁŹ
CH3COOCH2CH3+H2OŁŹ

 53ËæÌĂČâÏ”ÁĐŽđ°ž
53ËæÌĂČâÏ”ÁĐŽđ°ž
 ÓĐ»ú»ŻŃ§ÖȘʶÔÚÉú»îÖĐÓŠÓĂčă·șŁź
ÓĐ»ú»ŻŃ§ÖȘʶÔÚÉú»îÖĐÓŠÓĂčă·șŁź