
���� ��1�����ݸ���̼���Ƶ��ܽ��֪��121.2g����̼������Һ��̼���Ƶ�����Ϊ21.2g���ܼ���������100g���Ӷ���֪̼���Ƶ����ʵ������̶���������ɵ�̼�����Ƶ�������Ȼ�����ʣ����ܼ������������̼�����Ƶ��ܽ�ȣ��ʿ�������ܽ��̼�����Ƶ��������Ӷ��������������������
��2��2.24L������£�CO2��������ʵ���Ϊ0.1mol��������̼��ȫ��Ӧ��
��ֻ������CO2+2KOH=K2CO3+H2O��������0.1mol��K2CO3��������=0.1mol��138g/mol=13.8g��
��ֻ������CO2+KOH=KHCO3��������0.1mol��KHCO3��������=0.1mol��100g/mol=10g��
����13.8g��11.9g��10.0g�����Եõ��İ�ɫ������K2CO3��KHCO3�Ļ���
���ɫ������K2CO3 x mol��KHCO3 y mol������CԪ���غ㼰���������з��̼��㣬�ٸ��ݼ������غ����KOH���ʵ���������c=$\frac{n}{V}$����KOH��Һ���ʵ���Ũ�ȣ�
��3�����ݷ�Ӧ��֪����2molNaOH����ʱ����Һ����44g�������ɵ��������Ƶ����ʵ���Ϊxmol�����ݷ�Ӧ��⣻
��4���ɢڢۿ�֪��һ�����ļ����ҷ�Ӧʱ���ҵ���Խ�࣬���ɵij���Խ�٣�����ΪNaOH��Һ������ΪAlCl3��Һ�����ݢ��з���AlCl3+3NaOH�TAl��OH��3��+3NaCl�����������NaOH��Һ�����ʵ���Ũ�ȣ����ݢ��з���AlCl3+3NaOH�TAl��OH��3��+3NaCl��Al��OH��3+NaOH�TNaAlO2+2H2O��������������������������ɵij�������������AlCl3��Һ��Ũ�ȣ�
��� �⣺��1��20��ʱ������̼���Ƶ��ܽ��֪��121.2g����̼������Һ��̼���Ƶ�����Ϊ21.2g���ܼ���������100g��100gˮ���ܽ�̼�����Ƶ�����Ϊ9.6g��������ԭ���غ�֪������̼�����Ƶ�����=$\frac{21.2g}{106g/mol}$��2��84g/mol�T33.6g������Na2CO3+H2O+CO2=2NaHCO3֪��21.2g̼���Ʋμӷ�Ӧ��Ҫˮ������=$\frac{21.2g}{106g/mol}$��18g/mol=3.6g�������ܼ�������Ϊ100g-3.6g=96.4g��
�ܽ�̼�����Ƶ�����=$\frac{9.6g}{100g}$��96.4g=9.3g����������̼�����Ƶ�����=33.6g-9.3g=24.3g��
�ʴ�Ϊ��24.3��
��2��2.24L������£�CO2��������ʵ���Ϊ$\frac{2.24L}{22.4L/mol}$=0.1mol��
��ֻ������CO2+2KOH=K2CO3+H2O��������0.1mol��K2CO3��������=0.1mol��138g/mol=13.8g��
��ֻ������CO2+KOH=KHCO3��������0.1mol��KHCO3��������=0.1mol��100g/mol=10g��
����13.8g��11.9g��10.0g�����Եõ��İ�ɫ������K2CO3��KHCO3�Ļ���
���ɫ������K2CO3 x mol��KHCO3 y mol��
����̼ԭ���غ㣬�У�x mol+y mol=0.1 mol��
�ɶ���������֪��138g•mol-1��x mol+100 g•mol-1��y mol=11.9g
�������̣����x=0.05mol y=0.05mol
ԭ��Һ��KOH���ʵ���Ϊ 2xmol+ymol=2��0.05mol+0.05mol=0.15mol������KOH��Һ���ʵ���Ũ��Ϊ$\frac{0.15mol}{0.5L}$=0.3mol•L-1��
�ʴ�Ϊ��0.3��
��3�����ݷ�Ӧ��֪����2molNaOH����ʱ����Һ����44g�������ɵ��������Ƶ����ʵ���Ϊxmol�����У�
2Na+2H2O=2NaOH+H2��
2mol��m=44g
xmol 11g
����$\frac{2}{x}=\frac{44}{11}$
���x=0.5mol
�����ɵ��������Ƶ�����Ϊm=0.5mol��40g/mol=20g
����Һ��������=$\frac{20g}{100g}��100%=20%$��
�ʴ�Ϊ��20%��
��4���ɢڢۿ�֪��һ�����ļ����ҷ�Ӧʱ���ҵ���Խ�࣬���ɵij���Խ�٣�����ΪNaOH��Һ������ΪAlCl3��Һ��
�ڢ��з���AlCl3+3NaOH�TAl��OH��3��+3NaCl��1.56g���������ʵ���Ϊ$\frac{1.56g}{78g/mol}$=0.02mol����㣬��ȫ��Ӧ��
��NaOH�����ʵ���Ϊ0.02mol��3=0.06mol����NaOH��Һ�����ʵ���Ũ��Ϊ$\frac{0.06mol}{0.12L}$=0.5mol/L��
�ڢ��з���AlCl3+3NaOH�TAl��OH��3��+3NaCl��Al��OH��3+NaOH�TNaAlO2+2H2O����������������xmol����ʼ����������������������3xmol��
�����ܽ����������������������1��1��Ӧ���������0.02mol�������������������������ƣ�x-0.02��mol��
��һ��������������Ϊ3x+��x-0.02��=0.44L��0.5mol/L=0.22mol�����x=0.06mol�����������������0.06mol������ԭ��Һ��������0.06mol��
�Ȼ���Ũ��Ϊ$\frac{0.06mol}{0.12L}$=0.5mol/L��
�ʴ�Ϊ��AlCl3��0.5��NaOH��0.5��
���� ���⿼�黯ѧ����ʽ���йؼ��㣬Ϊ��Ƶ���㣬��ȷ�������������ǽⱾ��ؼ���֪��������������������Һ�μ�˳��ͬ��������ﲻͬ�����ؿ���ѧ������������������Ŀ�Ѷ��еȣ�


 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȡ��Na2CO3��Һʱ������ȡ�����࣬Ϊ�˲��˷ѣ��ְѹ������Լ������Լ�ƿ�� | |
| B�� | ���ܽ⡢���˵ķ��������Ȼ��ƺ�����صĻ���� | |
| C�� | ����NaOH���壬������ƽ�����ϷŴ�С���ֽƬ��Ȼ��NaOH��������ֽƬ�ϳ��� | |
| D�� | ���ö����ЧӦ����������Һ�ͽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
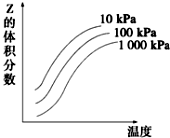 ͼ���¶Ⱥ�ѹǿ��X+Y?2Z��ӦӰ���ʾ��ͼ��ͼ�к������ʾ�¶ȣ��������ʾƽ��ʱ���������Z���������������������ȷ���ǣ�������
ͼ���¶Ⱥ�ѹǿ��X+Y?2Z��ӦӰ���ʾ��ͼ��ͼ�к������ʾ�¶ȣ��������ʾƽ��ʱ���������Z���������������������ȷ���ǣ�������| A�� | �������淴Ӧ������ӦΪ���ȷ�Ӧ | B�� | X��Y��Z��Ϊ��̬ | ||
| C�� | ����ѹǿƽ��������Ӧ�����ƶ� | D�� | ������Ӧ���淴Ӧ�ġ�H��0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ԭ�ӣ�${\;}_{1}^{2}$D | |
| B�� | S2-�Ľṹʾ��ͼ��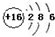 | |
| C�� | ������Ϊ53��������Ϊ78�ĵ�ԭ�ӣ�${\;}_{53}^{131}$I | |
| D�� | N2�ĵ���ʽ��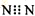 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Һ����FeBr3�������������屽 | |
| B�� | ��ϩ��������һ�������·�Ӧ����ClCH2CH=CH2�ķ�Ӧ | |
| C�� | ��ϩʹ���Ը��������Һ��ɫ | |
| D�� | ��ϩ��HCl���巴Ӧ����һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ú��ʯ���Dz���������Դ����Ȼ���ǿ�������Դ | |
| B�� | �ڻ�ѧ��Ӧ�У���Ӧ��ת��Ϊ�������ͬʱ����Ȼ���������ı仯 | |
| C�� | ��ֵָ��һ�������£�1mol��������ȫȼ�����ų������� | |
| D�� | ����ѧ�����жϿ���ѧ���ų������������γɻ�ѧ�������յ���������Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ӧ����������ԭ�������������� | B�� | B2H6���ȼ������B2O3��H2O | ||
| C�� | B2H6�д��ڹ��ۼ������Ӽ� | D�� | ÿ����1molB2H6ת��3mol���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
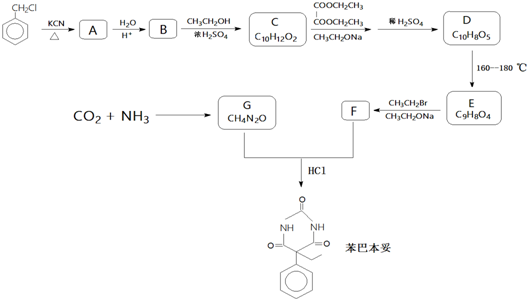
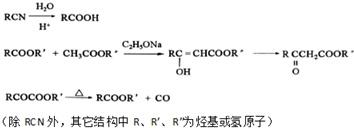
 ��E��F�ķ�Ӧ����ȡ����Ӧ
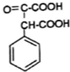
��E��F�ķ�Ӧ����ȡ����Ӧ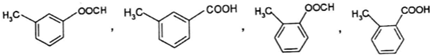 ������һ�֣���
������һ�֣��� �ͱ����ṹ��
�ͱ����ṹ��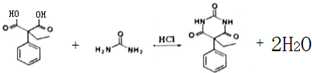 ��
��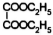 ���ĺϳ�·�ߣ�������ͼ��ʾ�����Լ���ѡ����
���ĺϳ�·�ߣ�������ͼ��ʾ�����Լ���ѡ�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com