
Ϊ�ⶨAlCl3��Һ�����ʵ���Ũ�ȣ�ijѧ����AlCl3��Һ���뵽20mL 0.3mol��L��1NaOH��Һ�У�����AlCl3��Һ �����V�����ɳ�����������g����ϵ����ͼ��ʾ����AlCl3 ��Һ�����ʵ���Ũ�ȼ�a���ֵӦΪ
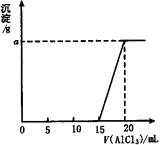
( )
A. 0.1mol��L��1��0.156g
B. 0.15mol��L��1��0.234g
C. 0.2mol��L��1��0.312g
D. 0.05mol��L��1��0.078g
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
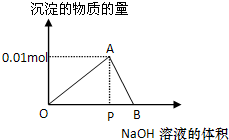 ��20mLij���ʵ���Ũ�ȵ�AlCl3��Һ�еμ�2mol/L NaOH��Һʱ����μ���NaOH��Һֱ�����������ⶨ�������NaOH��Һ�������mL�������ó��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����
��20mLij���ʵ���Ũ�ȵ�AlCl3��Һ�еμ�2mol/L NaOH��Һʱ����μ���NaOH��Һֱ�����������ⶨ�������NaOH��Һ�������mL�������ó��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�013
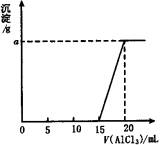
( )
A. 0.1mol��L��1��0.156g
B. 0.15mol��L��1��0.234g
C. 0.2mol��L��1��0.312g
D. 0.05mol��L��1��0.078g
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com