
��8�֣�(1) �õؿ���ij��ҪԪ�������Ķ��ֲ�Ʒ���ִ��߿Ƽ���ռ����Ҫλ�ã������ѧ���ִ�������������Ҫ���á����磺
�١������оƬ����Ҫ�ɷ��� ��(������)
�ڡ����ά����Ҫ�ɷ��� ��(������)
(2)���ǵؿ��к����ܸߵ�Ԫ�أ��䵥�ʺͻ����������ͺʹ�ͳ�ǽ�����������;�㷺
�١�д����ҵ����̼���ʻ�ԭ���������Ʊ���Ļ�ѧ��Ӧ����ʽ��
_________________________________________________________��
�ڡ��÷���ʽ��ʾΪʲô�������в��������Լ�ƿ��ż�����Һ
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A-F����Ԫ���У���A���Ϊ������Ԫ�أ����ǵ�ԭ�ӽṹ�����������ʾ��
A-F����Ԫ���У���A���Ϊ������Ԫ�أ����ǵ�ԭ�ӽṹ�����������ʾ��| ��� | Ԫ�� | �ṹ������ | ||
| �� | A | �����г����Ľ��������������Ȼ����Է����������35.5 | ||
| �� | B | ԭ���������������ڲ��������
| ||
| �� | C | �γɻ�������������Ԫ��֮һ���䵥��Ϊ���� | ||
| �� | D | �ؿ��к�������Ԫ�� | ||
| �� | E | ��Dͬ���� | ||
| �� | F | ��Eͬ���ڣ����������������ڵ��Ӳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��F����Ԫ���У���C��������Ϊ������Ԫ�أ����ǵ�ԭ�ӽṹ���������±���ʾ��
| Ԫ�� | �ṹ������ |
| A | ԭ���������������ڲ����������1/5 |
| B | �γɻ�������������Ԫ�أ��䵥��Ϊ���� |
| C | �����г����Ľ������������ֳ������Ȼ������Է����������35.5 |
| D | �ؿ��к�������Ԫ�� |
| E | ��Dͬ���� |
| F | ��Eͬ���ڣ����������������ڵ��Ӳ��� |
��ش��������⣺
��1��A��Ԫ�����ڱ��е�λ���� ��A��E�γɵĻ�����ĵ���ʽ�� ��
��2��C��ij���Ȼ����Ũ��Һ���Ը�ʴӡˢ��·���ϵĽ���ͭ���˷�Ӧ�����ӷ���ʽ�� ��
��3��B�ĵ�����D���⻯����һ�������·�Ӧ����BD����һ����Ļ�ѧ����ʽ�� ��
��4��F��������ˮ��Һ�����ԣ�ԭ���� �������ӷ���ʽ��ʾ����F�ĵ�����C��D�γɵ���Է�������Ϊ160�Ļ�������һ�������·�Ӧ�Ļ�ѧ����ʽ�� ��
��5��A��F�γɵĺϽ�����Ҫ�Ĺ�ҵ���ϡ�ijͬѧ��ʹ����ƽ����ͼ��ʾ��װ�ã����ԲⶨijЩ���ݼ�������úϽ���AԪ�صĺ�������װ�����������������������Բ��ƣ�

��ʵ����Ҫ�ⶨ�������������Ͻ������m�Լ�a��b��
a�� ��
b�� ��
�ںϽ���AԪ�ص����������� ���ú�m��a��b��ʽ�ӱ�ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�����ʦ���и�һ������ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��11�֣�
��1��A��B��C��DΪ���ֶ���������Ԫ�أ���ԭ����������������֪A������������������Ӳ�����2����B�ǵؿ��к�����ߵ�Ԫ�أ�Bԭ�ӵ�������������Dԭ��������������2����Cԭ�������ֻ��һ�����ӡ���Ԫ�ط���ΪA ,
B ��C ��D ��D������������Ӧˮ����Ļ�ѧʽΪ ��AB2 �ĵ���ʽΪ ��
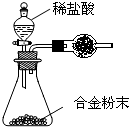
��2�������к��зḻ�ĵ⡣Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飬��ش��й����⡣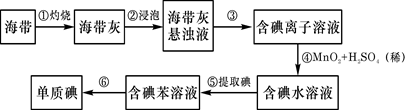
(��)��������պ���ʱ������Ҫ���żܡ��������������⣬����Ҫ�õ����������е� ��
A.�ձ� B. �ƾ��� C.������ D. ������
����������۵�ʵ����������� �������Ŀ���ǴӺ��ⱽ��Һ�з�
������ʵ�ͻ��ձ����ò����ʵ����������� ��
����������ܵķ�Ӧ�е����ӱ� �����������ԭ������
���������鵥�ʵ�ķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʦ���и�һ������ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��11�֣�
��1��A��B��C��DΪ���ֶ���������Ԫ�أ���ԭ����������������֪A������������������Ӳ�����2����B�ǵؿ��к�����ߵ�Ԫ�أ�Bԭ�ӵ�������������Dԭ��������������2����Cԭ�������ֻ��һ�����ӡ���Ԫ�ط���ΪA ,
B ��C ��D ��D������������Ӧˮ����Ļ�ѧʽΪ ��AB2 �ĵ���ʽΪ ��
��2�������к��зḻ�ĵ⡣Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飬��ش��й����⡣
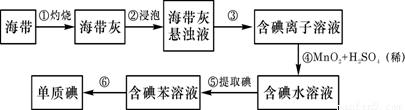
(��)��������պ���ʱ������Ҫ���żܡ��������������⣬����Ҫ�õ����������е� ��
A.�ձ� B. �ƾ��� C.������ D. ������
����������۵�ʵ����������� �������Ŀ���ǴӺ��ⱽ��Һ�з�
������ʵ�ͻ��ձ����ò����ʵ����������� ��
����������ܵķ�Ӧ�е����ӱ� �����������ԭ������
���������鵥�ʵ�ķ����� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com