

��1���Ƚ���NH4��2Cr2O7�þƾ��Ƽ��ȣ��շֽ�ʱ����ƾ��ƣ���NH4��2Cr2O7�����ֽ�����N2��H2O��Cr2O3���壬���巢�졣��������ȱ䰵ʱ����Ũ��ˮ�й����������ֱ�������ҿ�ʼ����ƿ�ڵ��ܿڸ�����������������ɫ���̣����ָ�������йػ�ѧ����ʽΪ
______________________________��______________________________��
______________________________��______________________________��
��2���������ƿ�п�����������ɫ�����塣ʵ�����Cr2O3�������δ�䡣д����NH4��2Cr2O7�ֽ�Ļ�ѧ����ʽ________________________________________________________��
��3��ʵ��֤���˰��Ĵ�������_______________��Ӧ������ȡ������ȡ��������ݵ�ʵ��������________________________________________________________________��
��Ӧ�Ĵ�����_______________��
��1��4NH3+5O2![]() 4NO+6H2O
4NO+6H2O
2NO+O2====2NO2 3NO2+H2O====2HNO3+NO HNO3+NH3====NH4NO3
��2����NH4��2Cr2O7![]() N2��+4H2O+Cr2O3
N2��+4H2O+Cr2O3
��3������ ����ƾ��ƺ�����ʯ�����Ϲ�����ȱ䰵ʱ����Ũ��ˮ�йĿ�����ʹ����������������Ӧ�������ֱ����
Cr2O3
����������Ӧע��֪ʶ��Ǩ�ƣ�NH3��������������Ϥ��֪ʶ������ͻ�ƿ���������NH3�Ĵ���������ȷCr2O3�����ó�Ϊ��������ۡ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)��AΪŨ���BΪ��3���ڽ���Ԫ�ص�Ƭ״���ʣ��ڳ���������ˮ��Ӧ��CΪƷ����Һ���۲쵽Ʒ����Һ��ɫ����B��_____________(д��ѧʽ)��B��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ____________________________����Ӧ�����ձ��м����ˮ���ֿɹ۲쵽�Թ�C�е�������_____________________________________________________________________��
(2)��BΪNa2CO3��Һ��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�C����Һ�ɳ������ǣ�����AӦ���е�������__________��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е�������_____________________________________________________________________��
(3)��B����ʯ�ң�ʵ���й۲쵽C��Һ���γɳ�����Ȼ������ܽ⡣������ǡ������ܽ��Ϊ������Һʱ���ر�E��Ȼ�����ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��__________(������)��C��__________(�ѧʽ)�������ǻ����Һ��Ӧ���÷�Ӧ�����ӷ���ʽΪ__________������D�ڴ�ʵ���е�������_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2��ijѧ���ж�SO2��Na2O2��Ӧ�����������ƣ�����Ϊ�����жϺ����𣿣������������������__________����Ҫ˵������___________________________________________��
��3����ͬѧ��ȷ����Ӧ���Ƿ����������ɣ���������ͼ��ʾװ�ý���ʵ�顣
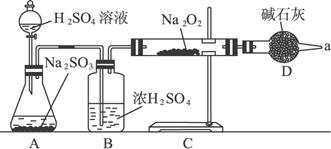
װ��B��������_________________________________________________��D�������ǣ�________________________________________��
��4��Ϊȷ�Ϸ�Ӧ���������±�������ʵ��
���� | ��������� |
��ȷ���Ƿ������������IJ����ǣ� |
|
��ȷ���Ƿ��������Ʋ����IJ����ǣ� |
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ������һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������ͼ��ʾװ�ý�������ʵ�飬ʵ������Ԥ�������һ�µ���
|
|
������� |
������� |
Ԥ��ٵ����� |
|
A |
����KI��Һ |
Ũ���� |
���������� |
|
B |
��̪��Һ |
Ũ���� |
���������� |
|
C |
AlCl3��Һ |
Ũ��ˮ |
�а�ɫ���� |
|
D |
ʪ���ֽ�� |
������ˮ |
��ֽ����ɫ |
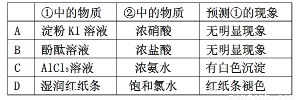
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijУѧ��������ͼ��ʾװ�ý���ʵ�飬��̽�������巢����Ӧ��ԭ���������ᴿ��Ӧ�IJ��
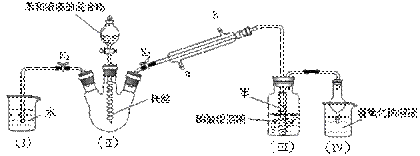
��ش��������⣺
��1��д��II�з�Ӧ�Ļ�ѧ����ʽ ��
��2���۲쵽II�е������� ��
��3��ʵ�鿪ʼʱ���ر�K2������K1�ͷ�Һ©���������μӱ���Һ��Ļ��Һ����Ӧ��ʼ��III��С�Թ��ڱ��������� ��
��4����˵������Һ�巢����ȡ����Ӧ�������� ��
��5����������ƿ�ڷ�Ӧ���Һ�����ν�������ʵ������Ϳɵõ��ϴ������屽��
�� ������ˮϴ�ӣ�����Һ���� ��5%��NaOH��Һϴ�ӣ�����Һ��
�� ������ˮϴ�ӣ�����Һ���� ������ˮCaCl2��ĩ���
�� ����������ƣ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com