
A��B��Ϊ���ε�ˮ��Һ��A�����ԣ�B�ʼ��Բ����������ԡ�����Ϊ���ʵ�鲽���ʵ������
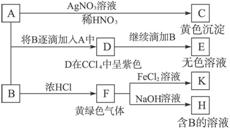
������������⣺
��1��д��A��B��C�Ļ�ѧʽ��A_____________��B_____________��C_____________��
��2������д��A��D��D��E��E�к���ij+5��Ԫ�صĺ���������ӣ������ӷ���ʽ��______________________________________��_______________________________________��
��3����SO2����ͨ��D��Һ��D��Һ��Ϊ��ɫ�����������ᡣд����Ӧ�����ӷ���ʽ��______________________________________________________________________________��
��4��д����F��H�Ļ�ѧ����ʽ��__________________________________________________��
��1��NaI NaClO AgI
(2)2I-+ClO-+H2O====I2+Cl-+2OH-
I2+5ClO-+2OH-====2![]() +5Cl-+H2O
+5Cl-+H2O
(3)I2+SO2+2H2O====2I-+![]() +4H+
+4H+
(4)Cl2+2NaOH====NaCl+NaClO+H2O
�������ͻ�ƿ�Ϊ�������ʵ���ɫ���ɴ˿�֪Ϊ±�ص��ʼ��仯����֮����ת�䣬����B�ʼ��Բ����������Լ�H�Ǻ�B����Һ�����Ƴ�AΪNaI��BΪNaClO��CΪAgI��DΪI2����2����NaClO����ǿ�����ԣ��ɰ�I2����ΪNaIO3����3��I2�ɰ�SO2����Ϊ![]() ����4��FHΪCl2��NaOH�ķ�Ӧ��
����4��FHΪCl2��NaOH�ķ�Ӧ��


 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д� ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| �¶� �ܽ�� �� |
0�� | 10�� | 20�� | 30�� | 40�� | 50�� |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʦ����2006��2007ѧ�����ѧ�ڸ����¿��Ծ�(��)����ѧ���� ���ͣ�022
| |||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��Ӹ�����3����ѧ������ѡһ������
1.�ۻ�ѧ����ѡ��ѧ�뼼����
��ش��ȼҵ�е��������⣺
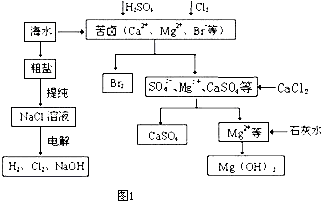
(1)�������ռ��ǵ��ʳ��ˮʱ���չ̶��ı���k(������)���ɵIJ�Ʒ��������k__________(Ҫ��������ʽ�ͽ��)��
(2)ԭ�ϴ����г�������ɳ��Ca2+��Mg2+��Fe3+�� �����ʣ����뾫�ƺ���ܹ����ʹ�á�����ʱ����������ˮ���˺�Ҫ������Լ��ֱ�Ϊ��Na2CO3����HCl(����)����BaCl2����3���Լ����ӵĺ���˳����_________(�����)��
�����ʣ����뾫�ƺ���ܹ����ʹ�á�����ʱ����������ˮ���˺�Ҫ������Լ��ֱ�Ϊ��Na2CO3����HCl(����)����BaCl2����3���Լ����ӵĺ���˳����_________(�����)��
(3)�ȼҵ�Ǹߺ��ܲ�ҵ��һ�ֽ�������ȼ�ϵ������ϵ��¹��տ��Խ�(��)��30%���ϡ������ֹ�������У�������ϵĴ�����ת����ϵ����ͼ��ʾ�����еĵ缫δ��������õ�����Ĥ��ֻ����������ͨ����

��ͼ��X��Y�ֱ���_________��_________(�ѧʽ)�������Ƚ�ͼʾ������������������a%��b%�Ĵ�С_________��
�ڷֱ�д��ȼ�ϵ��B�������������Ϸ����ĵ缫��Ӧ
������_________��������_________��
��������Ƶ���Ҫ��(��)��֮������(д��2��)
___________________________��___________________________��
2.�ۻ�ѧ����ѡ�����ʽṹ�����ʣ�
��֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӡ�X��Y���γɻ�����X2Y3��ZԪ�ؿ����γɸ�һ�����ӡ���ش��������⣺
(1)XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ_________����Ԫ�صķ���Ϊ_________��
(2)YԪ��ԭ�ӵļ۲���ӵĹ����ʾʽΪ_________����Ԫ�ص�������_________��
(3)X��Z���γɻ�����XZ3���û�����Ŀռ乹��Ϊ________________________��
(4)��֪������X2Y3��ϡ������Һ�пɱ�����п��ԭΪXZ3�����ﻹ��ZnSO4��H2O���÷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
(5)�Ƚ�X���⻯����ͬ��ڶ���������Ԫ�����γɵ��⻯���ȶ��ԡ��е�ߵͲ�˵������____________________________________��
3.�ۻ�ѧ����ѡ���л���ѧ������
A��J��Ϊ�л����������֮���ת������ͼ��ʾ��

ʵ�������
��D���ܷ���������Ӧ������������Ʒ�Ӧ�ų�������
�ں˴Ź������ױ���F�������������⣬��������֮��Ϊ1��1��1��
��G��ʹ������Ȼ�̼��Һ��ɫ��
��1 mol J�����������Ʒ�Ӧ�ɷų�22.4 L����(��״��)��
�����������Ϣ�ش��������⣺
(1)A�Ľṹ��ʽΪ_________(�����������칹)����A����B�ķ�Ӧ������_________��Ӧ��
(2)D�Ľṹ��ʽΪ___________________________��
(3)��E����F�Ļ�ѧ����ʽΪ____________��E�еĹ�������____________(������)����E������ͬ�����ŵ�E��ͬ���칹�廹��____________(д���ṹ��ʽ�������������칹)��
(4)G�Ľṹ��ʽΪ____________________________________��
(5)��I����J�Ļ�ѧ����ʽΪ________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com