
 ���������ҹ���������������������Ⱥ�ڵĸ߶ȹ��У�һ����Ϊϸ�������PM2.5��ֱָ��С�ڻ����2.5�Ŀ������ָ������������������أ����ͬʱ���⻯ѧ����Ҳ��ʼ̧ͷ�������ɷǼ�������NOx���ŷ�����ģ�����ʱ��ջ���ֲ�ɫ����������һ������Ⱦ������Ũ�������ǹ⻯ѧ�����γɵı�־����ش�����������⣺
���������ҹ���������������������Ⱥ�ڵĸ߶ȹ��У�һ����Ϊϸ�������PM2.5��ֱָ��С�ڻ����2.5�Ŀ������ָ������������������أ����ͬʱ���⻯ѧ����Ҳ��ʼ̧ͷ�������ɷǼ�������NOx���ŷ�����ģ�����ʱ��ջ���ֲ�ɫ����������һ������Ⱦ������Ũ�������ǹ⻯ѧ�����γɵı�־����ش�����������⣺| ʵ����� | c��NH4+��/mol•L-1 | c��NO2-��/mol•L-1 | ��/mol•L-1•s-1 |
| 1 | 0.0100 | 0.200 | 5.4��10-7 |
| 2 | 0.0200 | 0.200 | 1.08��10-6 |
| 3 | 0.200 | 0.040 | 2.16��10-6 |
| 4 | 0.200 | 0.060 | 3.24��10-6 |
���� ��1������������Ķ����ϴ𰸣�
��2��һ����������������Ӧ���ɶ������������������ܲ����������ԭ�ӣ�������һ�������ܷ�Ӧ���ɶ���������������
��3������Ħ��������ܶȵijɼ�Ϊƽ��Ħ������������ʮ����˷���N2O4��NO2�����ʵ����ȣ�
��4�����¡�����Ӧ��Ũ�ȣ������ѧ��Ӧ���ʣ����ݦ�=k•c��NH4+��x•c��NO2-��y���⣮
��� �⣺��1��a��ȼúȡů b�����ͽ�ͨ�豸 d����ɽ�緢 e���ֲ�ս�� f���ɿ�ҵ�ܲ���۳���������ϸ������ָ���ߣ��ʴ�Ϊ��abdef��
��2��һ����������������Ӧ���ɶ���������2NO+O2=2NO2�����������ܲ����������ԭ�ӣ�NO2=NO+O��������һ�������ܷ�Ӧ���ɶ���������������O3+NO=NO2+O2�������������γɹ⻯ѧ������15��00Ũ�Ƚϸߣ����ܳ��֣��ʴ�Ϊ��2NO+O2=2NO2��NO2=NO+O��O3+NO=NO2+O2��c��
��3��ƽ��Ħ������=24.5L/mol��3.18g/L=77.9g/mol������ʮ����˷���N2O4��NO2�����ʵ����ȣ�
N2O4 92 14.1
77.9
NO2 46 31.9
NO2��N2O4�����ʵ�����Ϊ��31.9��14.1���ֽ��N2O4Ϊ15.95���ܵ�N2O4Ϊ30.05��
N2O4�ķֽ���Ϊ$\frac{15.95}{30.05}$��
�ʴ�Ϊ��77.9g/mol��53.1%��
��4�����¡�����Ӧ��Ũ�ȣ������ѧ��Ӧ���ʣ�
��=k•c��NH4+��x•c��NO2-��y=k•c��NH4+��•c��NO2-����Ũ�ȱ�Ϊԭ����һ�룬����Ϊԭ����$\frac{1}{4}$���ʴ�Ϊ�����¡�����Ӧ��Ũ�ȣ�$\frac{1}{4}$��
���� ���⿼�黯ѧ����ʽ����д����㣬��Ŀ�Ѷ��еȣ�ע����������Ϣ���⣬����������ѧ�������õĿ�ѧ������


 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��NaH��H2O��Ӧ�Ļ�ѧ����ʽΪNaH+H2O�TNaOH+H2����
��NaH��H2O��Ӧ�Ļ�ѧ����ʽΪNaH+H2O�TNaOH+H2�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H${\;}_{2}^{18}$O��Ͷ��Na2O2���壺2H${\;}_{2}^{18}$O+2O${\;}_{2}^{2-}$�T4OH-+18O2�� | |
| B�� | ��0.1 mol•L-1��pH=1��NaHA��Һ�м���NaOH��Һ��H++OH-�TH2O | |
| C�� | �Խ�����Ϊ������ⱥ������ͭ��Һ��Cu2++2H2O�T2Cu+O2��+4H+ | |
| D�� | NH4Al��SO4��2��Һ�м���Ba��OH��2��ҺSO42-ʹ��ȫ������Al3++2SO42-+2Ba2++4OH-�TAlO2-+2BaSO4��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
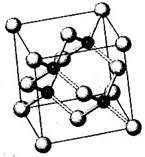 N��P��As��Ga��Cr��Ԫ�ػ���������࣬������Ҫ���о���ֵ��Ӧ�ü�ֵ����ش��������⣺
N��P��As��Ga��Cr��Ԫ�ػ���������࣬������Ҫ���о���ֵ��Ӧ�ü�ֵ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ͨ��ˮ�� Cl2+H2O?H++Cl-+HClO | |
| B�� | �������ȫȼ��CH4��g��+2O2��g��$\frac{\underline{\;��ȼ\;}}{\;}$ CO2��g��+2H2O��l����H��0 | |
| C�� | ���������ˮ��CH3COOH+H2O?CH3COO-+H3O+ | |
| D�� | ����ˮ������Ӧ 2Fe+3H2O$\frac{\underline{\;����\;}}{\;}$ Fe2O3+3H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3�� | B�� | 4�� | C�� | 5�� | D�� | 6�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 12�� | B�� | 11�� | C�� | 9�� | D�� | 7�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���õ���ʽ��ʾ����
���õ���ʽ��ʾ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com