
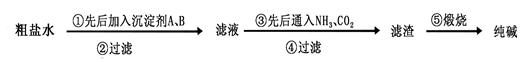
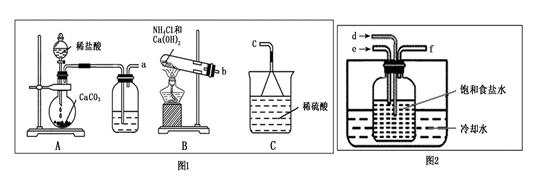
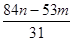 ��100%�����������𰸾��÷֣���2�֣� ��4�� c d��2�֣�
��100%�����������𰸾��÷֣���2�֣� ��4�� c d��2�֣� Na2CO3��H2O��CO2�� ��m��
Na2CO3��H2O��CO2�� ��m��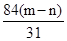 g ��m��n��g
g ��m��n��g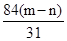 g��
g��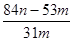 g
g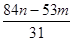 ��100%
��100%

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ���ᴿ������ | �����Լ� | ���뷽�� |
| A | �廯����Һ��NaI�� | ��ˮ��CCl4 | ��ȡ����Һ |
| B | �������ӣ� | NaOH��Һ | ���� |
| C | CO2��HCl�� | ����Na2CO3��Һ | ϴ�� |
| D | �Ҵ������ᣩ | ������ʯ�ң�CaO�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
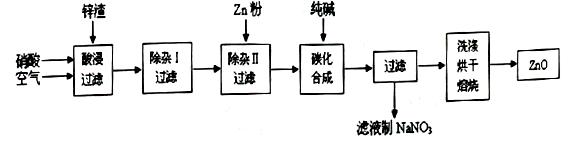
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 6.7 | 8.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֱ������ | B���ѻ����� | C���ƾ� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

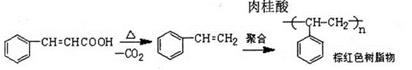
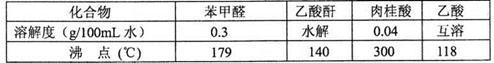
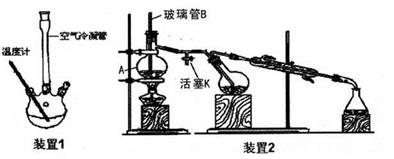
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B������ | C������ | D����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ȷ��� |
| B������������ʯ�ң���������ȩ���ټ���Ũ������������ |
| C������̼���ƺ�ͨ����ȡ�ķ������� |
| D������������Ӧ���з��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com