
��������Ҫ������Դ��������Ϊ�������õĹؼ��������ǵ�ǰ��ע���ȵ�֮һ��
(1)���������ȼ�ϣ���ȼ�ղ���Ϊ________��
(2)NaBH4��һ����Ҫ�Ĵ������壬����ˮ��Ӧ�õ�NaBO2���ҷ�Ӧǰ��B�Ļ��ϼ۲��䣬�÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________________����Ӧ����1 mol NaBH4ʱת�Ƶĵ�����ĿΪ________��
 (3)����ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⣺
(3)����ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⣺
 (g)
(g)  (g)��3H2(g)��
(g)��3H2(g)��
��ij�¶��£�������ܱ������м��뻷���飬����ʼŨ��Ϊa mol��L��1��ƽ��ʱ����Ũ��Ϊb mol��L��1���÷�Ӧ��ƽ�ⳣ��K��________��
(4)һ�������£���ͼ��ʾװ�ÿ�ʵ���л���ĵ绯ѧ����(���������л���)��
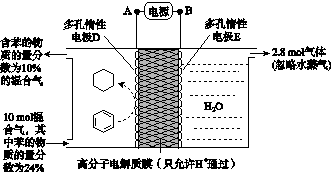
�ٵ����е����ƶ�����Ϊ________��(��A��D��ʾ)
������Ŀ�����ĵ缫��ӦʽΪ__________________��
�۸ô���װ�õĵ���Ч�ʦǣ�____________________��(�ǣ� ��100%������������С�����1λ)
��100%������������С�����1λ)
[��]��(1)H2O
(2)NaBH4��2H2O===NaBO2��4H2����
4NA��2.408��1024
(3)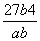 mol3��L��3
mol3��L��3
(4)��A��D����C6H6��6H����6e��===C6H12����64.3%[����] (2)BԪ�ط�Ӧǰ�ϼ۲��䣬������Ӧ��������NaBO2��֪����ӦǰNaBH4��B�Ļ��ϼ�Ҳ�ǣ�3�ۣ���HΪ��1�ۣ�ˮ����Ԫ�ػ��ϼ�Ϊ��1�ۣ��������з�Ӧ����������������Ԫ�ػ��ϼۿ�ȷ��ʧȥ��������(3)���ݻ�ѧ����ʽ��֪������H2Ϊ3b mol/L�����Ļ�����b mol/L����ƽ��ʱ������Ϊ(a��b) mol/L��������ʽȷ��ƽ�ⳣ����(4)�ٱ����ɻ������ǵ��ⷴӦ��Ϊ��ԭ��Ӧ�����缫D���������缫E�������������е����ƶ�����A��D����Ŀ������ǻ����飬������ͨ�����ӽ���Ĥ�������ƶ�����缫��ӦʽΪC6H6��6H����6e��===C6H12������������2.8 mol���壬��������OH���������ŵ������������ת�Ƶ���11.2 mol�����������ı������ʵ�����x mol��ͬʱ����x mol�����飬���缫��Ӧʽ��֪ת�Ƶĵ���Ϊ6x mol������ݵ����غ�֪��ͬʱ����������(11.2 mol��6x mol)��2��5.6 mol��3x mol�����ݻ������ɷֿ���ʽ 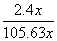 ��10%�����x��1.2����˴���װ�õĵ���Ч��Ϊ
��10%�����x��1.2����˴���װ�õĵ���Ч��Ϊ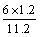 ��64.3%��
��64.3%��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ��䷴Ӧԭ��Ϊ
N2(g)��3H2(g)  2NH3(g)����H����92.4 kJ��mol��1��
2NH3(g)����H����92.4 kJ��mol��1��
һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�
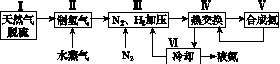
(1)��Ȼ���е�H2S���ʳ��ð�ˮ���գ�����ΪNH4HS��һ����������NH4HS��Һ��ͨ��������õ�������ʹ����Һ������д��������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(2)���������������ԭ�����£�
��CH4(g)��H2O(g)  CO(g)��3H2(g)
CO(g)��3H2(g)
��H����206.4 kJ��mol��1
��CO(g)��H2O(g)  CO2(g)��H2(g)
CO2(g)��H2(g)
��H����41.2 kJ��mol��1
���ڷ�Ӧ�٣�һ���������ƽ����ϵ��H2�İٷֺ��������ܼӿ췴Ӧ���ʵĴ�ʩ��____________��
a�������¶ȡ�b������ˮ����Ũ�ȡ�c�����������d������ѹǿ
���÷�Ӧ�ڣ���CO��һ��ת���������H2�IJ�������1 mol CO��H2�Ļ������(CO���������Ϊ20%)��H2O��Ӧ���õ�1.18 mol CO��CO2��H2�Ļ�����壬��CO��ת����Ϊ____________��
(3)ͼ(a)��ʾ500 �桢60.0 MPa�����£�ԭ����Ͷ�ϱ���ƽ��ʱNH3��������Ĺ�ϵ������ͼ��a�����ݼ���N2��ƽ�����������____________��
(4)�����¶ȶԺϳɰ���Ӧ��Ӱ�죬��ͼ(b)����ϵ�У�����һ�������µ��ܱ������ڣ���ͨ��ԭ������ʼ�����¶Ȳ������ߣ�NH3���ʵ����仯������ʾ��ͼ��
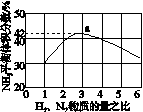 ��
��
(a)������������������(b)
(5)��������ͼ�У�ʹ�ϳɰ��ų��������õ�������õ���Ҫ������(�����)________����������������ߺϳɰ�ԭ����ת���ʵķ�����________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��
����ӵ�ص��ܷ�ӦΪ
LixC��Li1��xCoO2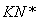 C��LiCoO2
C��LiCoO2
����ص��ܷ�Ӧ2Li��S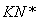 Li2S
Li2S
�й��������ֵ��˵����ȷ����(����)
A������ӵ�طŵ�ʱ�� Li����Ǩ��
B������س��ʱ��﮵缫������ԭ��Ӧ
C�����������ֵ�صı�������ͬ
D����ͼ��ʾ������ӵ�ظ�����س��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������(H3PO2)��һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԡ��ش��������⣺
(1)H3PO2��һ����ǿ�ᣬд������뷽��ʽ��_____________________________
________________________________________________________________________��
(2)H3PO2��NaH2PO2���ɽ���Һ�е�Ag����ԭΪ�����Ӷ������ڻ�ѧ������
��H3PO2�У�PԪ�صĻ��ϼ�Ϊ________��
������H3PO2���л�ѧ������Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ4��1������������Ϊ________(�ѧʽ)��
��NaH2PO2Ϊ________(����Ρ�����ʽ�Ρ�)������Һ��________(������ԡ������ԡ��������ԡ�)��
(3)H3PO2�Ĺ�ҵ�Ʒ��ǣ�������(P4)��Ba(OH)2��Һ��Ӧ����PH3�����Ba(H2PO2)2����������H2SO4��Ӧ��д��������Ba(OH)2��Һ��Ӧ�Ļ�ѧ����ʽ��____________________________________________��
(4)H3PO2Ҳ���õ��������Ʊ��������ҵ�������������ԭ����ͼ��ʾ(��Ĥ����Ĥ�ֱ�ֻ���������ӡ�������ͨ��)��
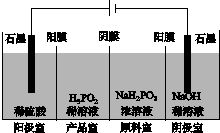
��д�������ĵ缫��Ӧʽ��_________________________________________��
�ڷ�����Ʒ�ҿɵõ�H3PO2��ԭ��_____________________________________
________________________________________________________________________��
�����ڲ��á����ҵ����������Ʊ�H3PO2���������ҵ����������������ҵ�ϡ������H3PO2ϡ��Һ���棬����ȥ���������Ʒ��֮�����Ĥ���Ӷ��ϲ������������Ʒ�ҡ���ȱ���Dz�Ʒ�л���________���ʣ������ʲ�����ԭ����________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ���ں����ø�ѹ������ػ����Ϸ�չ������һ�ֽ����⻯�������(MHNi���)�������й�˵������ȷ����(����)
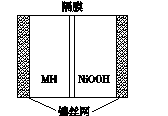
A���ŵ�ʱ������ӦΪNiOOH��H2O��e���D��Ni(OH)2��OH��
B����صĵ��Һ��ΪKOH��Һ
C�����ʱ������ӦΪMH��OH���D��H2O��M��e��
D��MH��һ�ഢ����ϣ������ܶ�Խ��ص������ܶ�Խ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й��Ȼ�ѧ����ʽ����д����Ӧ�ı�������ȷ����(����)
A���ܱ������У�9.6 g�����11.2 g���ۻ�ϼ�������17.6 g������ʱ���ų�19.12 kJ��������Fe(s)��S(s)===FeS(s)����H����95.6 kJ��mol��1
B��ϡ������0.1 mol��L��1 NaOH��Һ��Ӧ��H��(aq)��OH��(aq)===H2O(l)����H����57.3 kJ��mol��1
C����֪1 mol������ȫȼ������Һ̬ˮ���ų�������Ϊ285.5 kJ����ˮ�ֽ���Ȼ�ѧ����ʽΪ2H2O(l)===2H2(g)��O2(g)����H����285.5 kJ��mol��1
D����֪2C(s)��O2(g)===2CO(g)����H����221 kJ��mol��1�����֪C��ȼ���Ȧ�H����110.5 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Ϊ����ij��θ�����ڷ�ҩ�����������������������ĸ����� ��
A�����ԡ� B������ �� C����ԭ�� D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
.A��F����Ԫ���У���C��������Ϊ������Ԫ�أ����ǵ�ԭ�ӽṹ���������±���ʾ��
| Ԫ�� | �ṹ������ |
| A | ԭ���������������ڲ����������1/5 |
| B | �γɻ�������������Ԫ�أ��䵥��Ϊ���� |
| C | �����г����Ľ������������ֳ������Ȼ������Է����������35.5 |
| D | �ؿ��к�������Ԫ�� |
| E | ��Dͬ���� |
| F | ��Eͬ���ڣ����������������ڵ��Ӳ��� |
��ش��������⣺
(1)A��Ԫ�����ڱ��е�λ���ǡ���3���ڢ�A�塡��A��E�γɵĻ�����ĵ���ʽ�ǡ�Mg2��[

 ]2��������������21������ʦ��
]2��������������21������ʦ��
(2)C��ij���Ȼ����Ũ��Һ���Ը�ʴӡˢ��·���ϵĽ���ͭ���˷�Ӧ�����ӷ���ʽ��________________________________________________________________________
________________________________________________________________________��
(3)B�ĵ�����D���⻯����һ�������·�Ӧ����BD����һ����Ļ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(4)F��������ˮ��Һ�����ԣ�ԭ���ǡ�Al3����3H2O����Al(OH)3��3H����(�����ӷ���ʽ��ʾ)��F�ĵ�����C��D�γɵ���Է�������Ϊ160�Ļ�������һ�������·�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com