
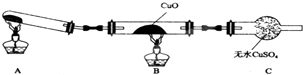
 ijŠ£ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§Ń§Ļ°ĶźµŖµÄÓŠ¹ŲĪļÖŹµÄŠŌÖŹÖ®ŗ󣬶ŌµŖŌŖĖŲµÄĒā»ÆĪļNH3ŠŌÖŹµÄĢ½¾æ£®
ijŠ£ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§Ń§Ļ°ĶźµŖµÄÓŠ¹ŲĪļÖŹµÄŠŌÖŹÖ®ŗ󣬶ŌµŖŌŖĖŲµÄĒā»ÆĪļNH3ŠŌÖŹµÄĢ½¾æ£®·ÖĪö £Ø1£©ĀČ»Æļ§ÓėĒāŃõ»ÆøĘŌŚ¼ÓČČĢõ¼žĻĀÉś³É°±Ęų”¢ĀČ»ÆøĘ”¢Ė®£»
£Ø2£©°±Ęų¼«Ņ×ČÜÓŚĖ®”¢ĀČ»Æļ§¶Ō°±ĘųŌŚĖ®ÖŠµÄČܽāÓ°Ļģ²»“ó£»
£Ø3£©¢ŁĀČ»Æļ§ŗĶĻūŹÆ»Ņ·“Ӧɜ³É°±ĘųŗĶĖ®£¬°±ĘųŗĶCuO·“Ó¦Ē°Ó¦ĻČøÉŌļ£»
¢Ś°±ĘųÓėCuO·“Ӧɜ³ÉĶ”¢µŖĘųŗĶĖ®£»
¢ŪŅĄ¾ŻŃõ»ÆŃĒĶŌŚĖįČÜŅŗÖŠ·¢Éś×ŌÉķŃõ»Æ»¹Ō·“Ӧɜ³ÉĶŗĶĶĄė×Ó£¬ČÜŅŗĄ¶É«Ö¤Ć÷Ńõ»ÆŃĒĶµÄ“ęŌŚ£®
½ā“š ½ā£ŗ£Ø1£©ĀČ»Æļ§ÓėĒāŃõ»ÆøĘŌŚ¼ÓČČĢõ¼žĻĀÉś³É°±Ęų”¢ĀČ»ÆøĘ”¢Ė®£¬·½³ĢŹ½ĪŖ2NH4Cl+Ca£ØOH£©2 $\frac{\underline{\;\;”÷\;\;}}{\;}$CaCl2+2NH3”ü+2H2O£¬
¹Ź“š°øĪŖ£ŗ2NH4Cl+Ca£ØOH£©2 $\frac{\underline{\;\;”÷\;\;}}{\;}$CaCl2+2NH3”ü+2H2O£»
£Ø2£©°±Ęų¼«Ņ×ČÜÓŚĖ®”¢ĀČ»Æļ§¶Ō°±ĘųŌŚĖ®ÖŠµÄČܽāÓ°Ļģ²»“ó£¬ĖłŅŌ²»ÄÜÓĆÓĆÅű„ŗĶĀČ»Æļ§ČÜŅŗµÄ·½·ØŹÕ¼Æ°±Ęų£¬
¹Ź“š°øĪŖ£ŗ·ń£»°±Ęų¼«Ņ×ČÜÓŚĖ®”¢ĀČ»Æļ§¶Ō°±ĘųŌŚĖ®ÖŠµÄČܽāÓ°Ļģ²»“ó£»
£Ø3£©¢ŁĀČ»Æļ§ŗĶĻūŹÆ»Ņ·“Ӧɜ³É°±ĘųŗĶĖ®£¬°±ĘųŗĶCuO·“Ó¦Ē°Ó¦ĻČøÉŌļ£¬¹Ź“š°øĪŖ£ŗŌŚ×°ÖĆAÓėBÖ®¼äŌö¼Ó×°ÓŠ¼īŹÆ»ŅµÄUŠĶ¹Ü£»
¢Ś°±ĘųÓėCuO·“Ӧɜ³ÉĶ”¢µŖĘųŗĶĖ®£¬»Æѧ·½³ĢŹ½£ŗ3CuO+2NH3$\frac{\underline{\;\;”÷\;\;}}{\;}$3Cu+N2+3H2O£¬¹Ź“š°øĪŖ£ŗ3CuO+2NH3$\frac{\underline{\;\;”÷\;\;}}{\;}$3Cu+N2+3H2O£»
¢ŪCu20ŹĒŅ»ÖÖ¼īŠŌŃõ»ÆĪļ£¬ŌŚĖįŠŌČÜŅŗÖŠCu+Éś³ÉCu+Cu2+£¬¾Ż“Ė·“Ó¦Éč¼ĘŹµŃéŃéÖ¤ŹĒ·ńŗ¬ÓŠŃõ»ÆŃĒĶ£¬²½ÖčĪŖ£ŗȔɣĮæѳʷ£¬¼ÓČėĻ”ĮņĖį£¬ČōČÜŅŗ³öĻÖĄ¶É«£¬ĖµĆ÷ŗ¬ÓŠCu2O£¬·ńŌņ²»ŗ¬ÓŠ£¬¹Ź“š°øĪŖ£ŗȔɣĮæѳʷ£¬¼ÓČėĻ”ĮņĖį£¬ČōČÜŅŗ³öĻÖĄ¶É«£¬ĖµĆ÷ŗ¬ÓŠCu2O£¬·ńŌņ²»ŗ¬ÓŠ£®
µćĘĄ ±¾Ģāæ¼²éĮĖ°±ĘųŹµŃéŹŅÖʱø·½·Ø£¬°±ĘųµÄŠŌÖŹŃéÖ¤ŹµŃéÉč¼ĘÓĆÓŚ·ÖĪöÅŠ¶Ļ£¬ŹģĻ¤ÖʱøŌĄķŹĒ½āĢā¹Ų¼ü£¬×¢ŅāŹµŃéÉč¼ĘµÄŗĻĄķŠŌ£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| µŚŅ»µēĄėÄÜ | Ąė×Ó°ė¾¶ | ČŪµć | ĖįŠŌ |
| N£¾O | O2-£¾Al3+ | KCl£¾Įņ»Ę | H2SO4£¼HClO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Č¼ĆŗŃĢĘųÖŠŗ¬ÓŠ“óĮæµÄµŖŃõ»ÆŗĻĪļ£ØNOx£©£¬²»ŅĖÖ±½ÓÅŷŵ½æÕĘųÖŠ£¬æɲÉÓĆŅŌĻĀ“ėŹ©¶ŌČ¼ĆŗŃĢĘų½ųŠŠ“¦Ąķ£®
Č¼ĆŗŃĢĘųÖŠŗ¬ÓŠ“óĮæµÄµŖŃõ»ÆŗĻĪļ£ØNOx£©£¬²»ŅĖÖ±½ÓÅŷŵ½æÕĘųÖŠ£¬æɲÉÓĆŅŌĻĀ“ėŹ©¶ŌČ¼ĆŗŃĢĘų½ųŠŠ“¦Ąķ£®| ĪĀ¶Č | Ź±¼ä/min n/mol | 0 | 10 | 20 | 40 | 50 |
| T1 | n£ØCH4£© | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
| T2 | n£ØCH4£© | 0.50 | 0.30 | 0.18 | 0.15 | 0.15 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | +6 | B£® | +3 | C£® | +4 | D£® | +2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | SO42Ņ» | B£® | Cu2+ | C£® | Ag+ | D£® | NO${\;}_{3}^{-}$ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na2CO3ČÜŅŗ | B£® | Na2SiO3ČÜŅŗ | C£® | NaOHČÜŅŗ | D£® | NaHSO3ČÜŅŗ”¢ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | FeCl2 | B£® | Fe3O4 | C£® | Fe£ØOH£©3 | D£® | Fe2£ØSO4£©3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ņ»¶Ø²»“ęŌŚBa2+£¬NH4+æÉÄÜ“ęŌŚ | B£® | CO32-Ņ»¶Ø“ęŌŚ | ||
| C£® | Na+Ņ»¶Ø“ęŌŚ | D£® | Ņ»¶Ø²»“ęŌŚCl- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
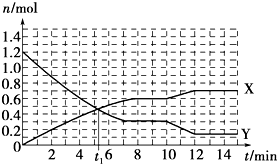
| A£® | t1minŹ±Õż”¢Äę·“Ó¦ĖŁĀŹĻąµČ | |
| B£® | XĒśĻß±ķŹ¾NH3µÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆµÄ¹ŲĻµ | |
| C£® | 0”«8 min£¬H2µÄĘ½¾ł·“Ó¦ĖŁĀŹv£ØH2£©=0.75 mol•L-1•min-1 | |
| D£® | 10”«12 min£¬N2µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖv£ØN2£©=0.25mol•L-1•min-1 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com