
 2NH3(g) ��H����92 kJ/mol
2NH3(g) ��H����92 kJ/mol ����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ��
����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ��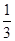 ��33.3% ��3���� �� �� ��
��33.3% ��3���� �� �� �� 2NH3(g)
2NH3(g)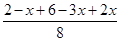 ��0.75
��0.75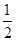 ��100%��50%
��100%��50%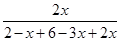 =
=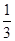
 2NH3(g)��֪������Ӧ�������С�Ŀ��淴Ӧ��������������ݻ����䣬��ѹǿ���ͣ���˵���ڷ�Ӧ�������������е�ѹǿʼ�մ��ڼ������е�ѹǿ��ѹǿ��Ӧ���ʿ죬����ƽ���ʱ���٣�������ƽ�������ʱ�䣺�ף��ҡ�
2NH3(g)��֪������Ӧ�������С�Ŀ��淴Ӧ��������������ݻ����䣬��ѹǿ���ͣ���˵���ڷ�Ӧ�������������е�ѹǿʼ�մ��ڼ������е�ѹǿ��ѹǿ��Ӧ���ʿ죬����ƽ���ʱ���٣�������ƽ�������ʱ�䣺�ף��ҡ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Q1 +Q2 +Q3���������� | B��0��5��Q1 +Q2 +Q3�� |
| C��0��5Q1 �C0��5Q2 +0��5Q3������ | D��1��5Q1 �C0��5Q2 +0��5Q 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)=ZnO(s)����H1����351.1 kJ��mol��1
O2(g)=ZnO(s)����H1����351.1 kJ��mol��1 O2(g)=HgO(s)����H2����90.7 kJ��mol��1
O2(g)=HgO(s)����H2����90.7 kJ��mol��1| A����441.8 kJ��mol��1 | B����254.6 kJ��mol��1 |
| C����438.9 kJ��mol��1 | D����260.4 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 3N2��2X��4H2O
3N2��2X��4H2O �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)=H2O(l) ��H����285.8 kJ��mol��1
O2(g)=H2O(l) ��H����285.8 kJ��mol��1| A��483.6 kJ | B��88 kJ | C��285.8 kJ | D��44 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com