
��ϩ��ʯ���ѽ�������Ҫ�ɷ֣�����ش��������⡣
(1)��ϩ�ĵ���ʽ____________���ṹ��ʽ____________��
(2)����������ϩ���Լ���______(�����)��
A��ϡ���� B��������Ȼ�̼��Һ
C��ˮ D�����Ը��������Һ
(3)���������У�����ͨ����ϩ�ӳɷ�Ӧ�õ�����______(�����)��
A��CH3CH3 B��CH3CHCl2
C��CH3CH2OH  D��CH3CH2Br
D��CH3CH2Br
(4)��֪ 2CH3CHO��O2 2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·������ͼ��ʾ��
 |
��Ӧ�ڵĻ�ѧ����ʽΪ____________________________________��
��ҵ������ϩΪԭ�Ͽ�������һ����Ҫ�ĺϳ��л��߷��ӻ�����䷴Ӧ�Ļ�ѧ����ʽΪ____________________________________����Ӧ������__________________��
 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������һ�����ᣬ��������ʴ��������֪25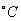 ʱ��
ʱ��

��20Ml0.lmol/L��������еμ�0.lmol/L��NaOH V mL������˵����ȷ����
A�������ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ��
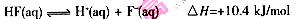
B����V=20 mLʱ����Һ�У�
C����V=20 mLʱ����Һ�У�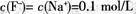
D����v>0ʱ����Һ��һ������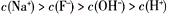
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ������һ�ַ�Ӧԭ�����£�
CH3OH(g)��H2O(g)===CO2(g)��3H2(g)
��H��49.0 kJ·mol��1
����˵����ȷ���� (����)
A��1 L CH3OH������1 Lˮ������Ӧ����1 L CO2������3 L������������49.0 kJ
B��1��CH3OH������1��ˮ���ӷ�Ӧ����1��CO2������3��H2��������49.0 kJ����
C����ͬ������1 mol CH3OH(g)��1 mol H2O(g)�������ܺ�С��1 mol CO2(g)��3 mol H2(g)�������ܺ�
D��1 mol CH3OH������1 molҺ̬ˮ��Ӧ����1 mol CO2������3 mol�������յ�����С��49.0 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������У�һ�ȴ���ֻ��һ�ֵ��� ( )
A��CH3 CH2 CH2 CH2CH3 B��CH3CH2CH3 C�� CH3CH2CH2CH3 D��C(CH3)4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����β���е��ж�����NO��CO����һ�������¿ɷ�����Ӧ����N2��CO2�����й��ڴ˷�Ӧ��˵���У���ȷ����( )
A����Сѹǿ������Ӧ���� B������ѹǿ�ܼ�С��Ӧ����
C��ʹ���ʵ��Ĵ���������Ӧ���� D�������¶ȶԷ�Ӧ������Ӱ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijȲ����������õ�2-�����飬��Ȳ���ǣ� ��
A.2-��-1-��Ȳ B.2-��-3-��Ȳ C.3-��-1-��Ȳ D.3-��-2-��Ȳ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1,3������ϩ��2����Ȳ�ֱ���������Ӧ���Ȼ�ѧ����ʽ���£�
CH2=CH��CH=CH2(g)��2H2(g)��CH3CH2CH2CH3(g)��236.6kJ
CH3��C��C��CH3(g)��2H2(g)��CH3CH2CH2CH3(g)��272.7kJ
�ɴ˲����ж�
A��1,3������ϩ��2����Ȳ�ȶ��Ե���Դ�С
B��1,3������ϩ��2����Ȳ���Ӵ�����������Ըߵ�
C��1,3������ϩ��2����Ȳ�ת������ЧӦ
D��һ��̼̼�����ļ���������̼̼˫���ļ���֮�͵Ĵ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�Ͽ������̿���Ҫ�ɷ���MnO2���ͻ�������Ҫ�ɷ���FeS2��Ϊ��Ҫԭ���Ʊ������ܴ��Բ���̼���̣�MnCO3�����乤ҵ�������£�

�ش��������⣺
��1��Ϊ������ܽ�������ԭ�ϵĽ����ʣ����Բ�ȡ�Ĵ�ʩ�У�дһ���� ��
��2�����������У��ڼ���ʯ�ҵ�����Һ��pHǰ���������������̿��������ǣ������ӷ���ʽ��ʾ�� ��
��3�����������Ŀ���dz�ȥ��Һ�е�Cu2+��Ca2+�����ʡ��������Һ��c(F-)=0.01 mol•L-1����Һ�в�����c(Ca2+)= ����֪��Ksp(CaF2)=1.46��10-10����
 ��4�����̹����У�298K��c(Mn2+)Ϊ1.05 mol•L-1ʱ��ʵ����MnCO3�IJ�������ҺpH����Ӧʱ��Ĺ�ϵ����ͼ��ʾ������ͼ����Ϣ�ó��Ľ����� ��
��4�����̹����У�298K��c(Mn2+)Ϊ1.05 mol•L-1ʱ��ʵ����MnCO3�IJ�������ҺpH����Ӧʱ��Ĺ�ϵ����ͼ��ʾ������ͼ����Ϣ�ó��Ľ����� ��
��5�����̹�������CO2���ɣ�������MnCO3�����ӷ���ʽ��___________ ��
��6���ӳ��̹����еõ�����MnCO3�IJ���������
�����̹���������֤��MnCO3�Ѿ�ϴ�Ӹɾ�
������ƷA�Ļ�ѧʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com