
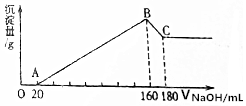
 ½«Ņ»¶ØÖŹĮæµÄĆ¾”¢ĀĮŗĻ½š£¬Ķ¶Čė200mLŅ»¶ØÅØ¶ČµÄŃĪĖįÖŠ£¬ŗĻ½šĶźČ«Čܽā£¬ĻņĖłµĆČÜŅŗÖŠµĪ¼Ó5mol/L NaOHČÜŅŗ£¬Éś³ÉµÄ³ĮµķÖŹĮæÓė¼ÓČėNaOHČÜŅŗĢå»żČēĶ¼ĖłŹ¾£®
½«Ņ»¶ØÖŹĮæµÄĆ¾”¢ĀĮŗĻ½š£¬Ķ¶Čė200mLŅ»¶ØÅØ¶ČµÄŃĪĖįÖŠ£¬ŗĻ½šĶźČ«Čܽā£¬ĻņĖłµĆČÜŅŗÖŠµĪ¼Ó5mol/L NaOHČÜŅŗ£¬Éś³ÉµÄ³ĮµķÖŹĮæÓė¼ÓČėNaOHČÜŅŗĢå»żČēĶ¼ĖłŹ¾£®·ÖĪö £Ø1£©ŃĪĖį¹żĮæÓėĒāŃõ»ÆÄĘ·“Ӧɜ³ÉĀČ»ÆÄĘŗĶĖ®£»
£Ø2£©ÓÉĶ¼æÉÖŖ£¬“Ó¼ÓČė20mLĒāŃõ»ÆÄĘČÜŅŗæŖŹ¼²śÉś³Įµķ£¬¼ÓČėĒāŃõ»ÆÄĘČÜŅŗĪŖ160mLŹ±£¬³ĮµķĮæ×ī“󣬓ĖŹ±ĪŖMg£ØOH£©2ŗĶAl£ØOH£©3£¬¼ĢŠųµĪ¼ÓĒāŃõ»ÆÄĘ£¬Al£ØOH£©3£¬Čܽā·“Ӧɜ³ÉĘ«ĀĮĖįÄĘŗĶĖ®£»²¢ÓÉAl£ØOH£©3+NaOH=NaAlO2+2H2O£¬¾ŻĘäÖŠĒāŃõ»ÆÄʵÄĮæĒóĒāŃõ»ÆĀĮµÄĮ棬²¢øł¾ŻŌŖĖŲŹŲŗć£¬µĆµ½ĀĮµÄĪļÖŹµÄĮ棬ŌŁĄūÓĆm=nM¼ĘĖćĀĮµÄÖŹĮ棻
£Ø3£©Bµć“¦£¬ČÜŅŗÖŠ“ĖŹ±“ęŌŚµÄČÜÖŹŹĒNaCl£»¼ÓČėĒāŃõ»ÆÄĘČÜŅŗĪŖ140mLŹ±£¬³ĮµķĮæ×ī“󣬓ĖŹ±ĪŖMg£ØOH£©2ŗĶAl£ØOH£©3£¬ČÜŅŗĪŖĀČ»ÆÄĘČÜŅŗ£¬øł¾ŻÄĘŌŖĖŲŹŲŗć“ĖŹ±ČÜŅŗÖŠn£ØNaCl£©=n£ØNaOH£©£¬¾Ż“Ė¼ĘĖć³ön£ØHCl£©£¬ŌŁĄūÓĆc=$\frac{n}{V}$¼ĘĖćŃĪĖįµÄĪļÖŹµÄĮæÅØ¶Č£®
½ā“š ½ā£ŗ£Ø1£©OA¶Ī£¬Ć»ÓŠ³ĮµķÉś³ÉµÄŌŅņŹĒ£¬ŃĪĖį¹żĮæÓėĒāŃõ»ÆÄĘ·“Ӧɜ³ÉĀČ»ÆÄĘŗĶĖ®£¬¹ŹĄė×Ó·½³ĢŹ½ĪŖH++OH-=2H2O£¬¹Ź“š°øĪŖ£ŗH++OH-=2H2O£»
£Ø2£©Ķ¼ÖŠBC¶Ī£¬³ĮµķĮæ¼õÉŁµÄŌŅņŹĒ£¬Al£ØOH£©3Čܽā·“Ӧɜ³ÉĘ«ĀĮĖįÄĘŗĶĖ®£¬¹Ź»Æѧ·½³ĢŹ½ĪŖAl£ØOH£©3+NaOH=NaAlO2+2H2O£¬¹ŹĄė×Ó·½³ĢŹ½ĪŖAl£ØOH£©3+OH-=AlO-2+2H2O£»
Al£ØOH£©3+NaOH=NaAlO2+2H2O
1 1
0.1 0.02L”Į5mol/L
Ōņ£ŗn£ØAl£ØOH£©3£©=0.1mol£¬øł¾ŻĀĮŌ×ÓŹŲŗć£¬n£ØAl£©=n£ØAl£ØOH£©3£©=0.1mol£¬¹Źm£ØAl£©=0.1mol”Į27g/mol=2.7g£¬
¹Ź“š°øĪŖ£ŗAl£ØOH£©3+OH-=AlO-2+2H2O£»2.7£»
£Ø3£©¾ŻŅŌÉĻ·ÖĪöµĆBµć“¦£¬ČÜŅŗÖŠ“ĖŹ±“ęŌŚµÄČÜÖŹŹĒNaCl£»¼ÓČėĒāŃõ»ÆÄĘČÜŅŗĪŖ160mLŹ±£¬³ĮµķĮæ×ī“󣬓ĖŹ±ĪŖMg£ØOH£©2ŗĶAl£ØOH£©3£¬ČÜŅŗĪŖĀČ»ÆÄĘČÜŅŗ£¬øł¾ŻÄĘŌŖĖŲŹŲŗć“ĖŹ±ČÜŅŗÖŠn£ØNaCl£©=n£ØNaOH£©=0.16L”Į5mol/L=0.8mol£¬øł¾ŻĀČŌŖĖŲŹŲŗćn£ØHCl£©=0.8mol£¬¹ŹŃĪĖįµÄĪļÖŹµÄĮæÅضČĪŖ$\frac{0.8mol}{0.2L}$=4mol/L£¬
¹Ź“š°øĪŖ£ŗNaCl£»4£®
µćĘĄ ±¾Ģāæ¼²éĆ¾ĀĮ»ÆŗĻĪļŠŌÖŹ”¢»ģŗĻĪļµÄ¼ĘĖć£¬ŅŌĶ¼ĻóĢāµÄŠĪŹ½æ¼²é£¬ĢāÄæÄѶČÖŠµČ£¬·ÖĪöĶ¼Ļóø÷½×¶ĪµÄ·¢ÉśµÄ·“Ó¦ŹĒ½āĢā¹Ų¼ü£¬ŌŁĄūÓĆŹŲŗć¼ĘĖć£¬ŹŌĢāÅąŃųĮĖѧɜµÄ·ÖĪöÄÜĮ¦¼°»Æѧ¼ĘĖćÄÜĮ¦£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃé²Ł×÷¼°ĻÖĻó | ŹµŃé½įĀŪ |
| A | ĻņijČÜŅŗÖŠ¼ÓČėŃĪĖįĖį»ÆµÄĀČ»Æ±µČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É | øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠSO42- |
| B | ½«Fe£ØNO3£©2ѳʷČÜÓŚĻ”H2SO4£¬µĪ¼ÓKSCNČÜŅŗČÜŅŗ±äŗģ | Fe£ØNO3£©2ѳʷŅŃŃõ»Æ±äÖŹ |
| C | ĻņŹ¢ÓŠÉŁĮæNaHCO3µÄŹŌ¹ÜÖŠµĪ¼Ó²ŻĖįČÜŅŗÓŠĘųÅŻ²śÉś | ĖįŠŌ£ŗ²ŻĖį£¾Ģ¼Ėį |
| D | ĻņÉŁĮæijĪļÖŹµÄĻ”ČÜŅŗÖŠµĪ¼ÓĻ”ŃĪĖį£¬²śÉśĮĖÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×ĒµÄĘųĢå | øĆĪļÖŹŅ»¶ØŹĒĢ¼ĖįŃĪ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ó¦ŗ¬ÓŠa | B£® | Ö»ŗ¬ÓŠb | C£® | ±Čŗ¬ÓŠc | D£® | Ņ»¶ØÓŠa”¢b”¢c |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĘųĢåµÄĆÜ¶Č²»±äŹ±£®ĖµĆ÷“ļµ½ĮĖĘ½ŗāדĢ¬ | |
| B£® | ¼ÓČėÉŁĮæµÄX£¬”÷H±ä“ó | |
| C£® | ¼ÓČėŅ»¶ØĮæµÄ¶čŠŌĘųĢå£¬Ę½ŗāĻņ×óŅĘ¶Æ | |
| D£® | ¼ÓČėÉŁĮæµÄYÕż·“Ó¦ĖŁĀŹ¼Óæģ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 3£ŗ7 | B£® | 7£ŗ1 | C£® | 5£ŗ3 | D£® | 1£ŗ1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 200mL | B£® | 300mL | C£® | 490mL | D£® | 720mL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ČõĖįµÄµēĄė³£ŹżŌ½Š”£¬ĘäĖł²śÉśµÄŃĪĖ®½āµÄ³Ģ¶ČŌ½“ó | |
| B£® | ČÜŅŗÖŠ·¢ÉśµÄĖ®½ā·“Ó¦ŹĒA-+H2O?HA+OH- | |
| C£® | ÓŠ¹ŲĄė×ÓÅØ¶Č“óŠ”¹ŲĻµŹĒc£ØM+£©£¾c£ØA-£©£¾c£ØOH-£©£¾c£ØH+£© | |
| D£® | øĆČÜŅŗŹĒĖįŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś¢Ū | B£® | ¢Ś¢Ż | C£® | ¢Ś¢Ü¢Ż | D£® | ¢Ś¢Ū¢Ü |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com