
”¾ĢāÄæ”æÓŠ¹ŲĪļÖŹµÄĮæµÄ¼ĘĖćŹĒ֊ѧ»ÆѧµÄÖŲŅŖ²æ·Ö£¬Ēė»Ų“šĻĀĮŠÓŠ¹ŲĪļÖŹµÄĮæµÄ¼ĘĖćĪŹĢā”£
£Ø1£©ŌŚ±ź×¼×“æöĻĀ£¬67.2 L CO2ŹĒ__________mol£¬ÖŹĮæĪŖ_______g£¬ŗ¬ÓŠ__________øöCO2·Ö×Ó£¬ĘäÖŠŗ¬ÓŠ__________molŃõŌ×Ó”£
£Ø2£©ŌŚ±ź×¼×“æöĻĀ£¬1.7 g°±ĘųĖłÕ¼µÄĢå»żŌ¼ĪŖ_________L£¬ĖüÓėĶ¬Ģõ¼žĻĀ_____mol H2Sŗ¬ÓŠĻąĶ¬µÄĒāŌ×ÓŹż”£
£Ø3£©Ä³ĘųĢ¬Ńõ»ÆĪļ»ÆѧŹ½ĪŖRO2£¬ŌŚ±ź×¼×“æöĻĀ£¬1.28 gøĆŃõ»ÆĪļµÄĢå»żŹĒ448 mL£¬ŌņŃõ»ÆĪļµÄĦ¶ūÖŹĮæĪŖ_______£¬RµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ__________”£
£Ø4£©ŹµŃéŹŅ³£ÓĆÅØĮņĖįµÄÖŹĮæ·ÖŹżĪŖ98%£¬ĆܶČĪŖ1.80 g”¤mL1£¬ĘäĪļÖŹµÄĮæÅØ¶ČŹĒ_______”£
£Ø5£©±ź×¼×“æöĻĀ£¬½«V L AĘųĢå£ØĦ¶ūÖŹĮæĪŖM g/mol£©ĶźČ«ČÜÓŚ0.1 LĖ®£ØĆܶČ1 g/cm3£©ÖŠ£¬ĖłµĆČÜŅŗµÄĆܶČĪŖd g/mL£¬Ōņ“ĖČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ_______mol/L”£
A£®![]() B£®
B£®![]() C£®
C£®![]() D£®
D£®![]()
”¾“š°ø”æ3.0 132 3NA 6 2.24 0.15 64 g/mol 32 18.4 mol/L B
”¾½āĪö”æ
£Ø1£©øł¾Żn=V/Vm¶žŃõ»ÆĢ¼µÄĪļÖŹµÄĮ棬øł¾Żm=nM¼ĘĖćĘäÖŹĮ棬øł¾ŻN=nNA¼ĘĖć·Ö×ÓŹżÄ棬ŃõŌ×ÓĪļÖŹµÄĮæĪŖ¶žŃõ»ÆĢ¼µÄ2±¶£»
£Ø2£©øł¾Żn=m/M¼ĘĖć°±ĘųĪļÖŹµÄĮ棬øł¾ŻV=nVm¼ĘĖć°±ĘųĢå»ż£¬øł¾ŻHŌ×ÓŹżÄæĻąµČ¼ĘĖćĮņ»ÆĒāµÄĪļÖŹµÄĮ棻
£Ø3£©øł¾Żn=V/VmŃõ»ÆĪļµÄĪļÖŹµÄĮ棬øł¾ŻM=m/n¼ĘĖćŃõ»ÆĪļµÄĦ¶ūÖŹĮ棬½ų¶ų¼ĘĖćRµÄĻą¶ŌŌ×ÓÖŹĮ森
£Ø4£©øł¾Żc=1000¦Ńw/M¼ĘĖćøĆÅØĮņĖįµÄĪļÖŹµÄĮæÅØ¶Č£»
£Ø5£©øł¾Żn=V/22.4L”¤mol£1¼ĘĖ江æöĻĀVLøĆĘųĢåµÄĪļÖŹµÄĮ棬ŌŁĄūÓĆm=nM¼ĘĖćøĆĘųĢåµÄÖŹĮ棬ČܼĮŗĶČÜÖŹµÄÖŹĮæŗĶĪŖČÜŅŗµÄÖŹĮæ£¬Č»ŗóĄūÓĆV=m/¦Ń¼ĘĖćČÜŅŗµÄĢå»ż£¬×īŗóĄūÓĆc=n/V¼ĘĖćøĆČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č£®
£Ø1£©¶žŃõ»ÆĢ¼µÄĪļÖŹµÄĮæĪŖ67.2L/22.4L”¤mol£1=3mol£»
ĘäÖŹĮæĪŖ3mol”Į44g”¤mol£1=132g£»
¶žŃõ»ÆĢ¼·Ö×ÓŹżÄæĪŖ3mol”Į6.02”Į1023mol£1=1.806”Į1024£»
ŃõŌ×ÓĪļÖŹµÄĮæĪŖ3mol”Į2=6mol£»
£Ø2£©1.7g °±ĘųĪļÖŹµÄĮæĪŖ1.7g/17g”¤mol£1=0.1mol£¬°±ĘųĢå»żĪŖ0.1mol”Į22.4L”¤mol£1=2.24L£¬
Óėŗ¬ÓŠĻąĶ¬HŌ×ÓŹżÄæµÄĮņ»ÆĒāµÄĪļÖŹµÄĮæĪŖ0.1mol”Į3/2=0.15mol£»
£Ø3£©Ńõ»ÆĪļµÄĪļÖŹµÄĮæĪŖ0.448L/22.4L”¤mol£1=0.02mol£¬Ńõ»ÆĪļµÄĦ¶ūÖŹĮæĪŖ1.28g/0.02mol=64g”¤mol£1£¬RµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ64-32=32£®
£Ø4£©ĆܶČĪŖ1.84g”¤cm£3”¢ÖŹĮæ·ÖŹżĪŖ 98% µÄÅØĮņĖį£¬ĘäĪļÖŹµÄĮæÅضČ=1000”Į1.84”Į98%/98mol”¤L£1=18.4 mol”¤L£1£»
£Ø5£©±ź×¼×“æöĻĀ£¬ĘųĢåµÄĪļÖŹµÄĮæĪŖVL/22.4L”¤mol£1=V/22.4mol£¬øĆĘųĢåµÄÖŹĮæĪŖ£ŗV/22.4mol”ĮM g”¤mol£1=VM/22.4g£¬0.1LĖ®µÄÖŹĮæĪŖ£ŗ100mL”Į1g”¤mL£1=100g£¬ŌņČÜŅŗµÄÖŹĮæĪŖ£ŗVM/22.4g+100g£¬ĖłŅŌøĆČÜŅŗµÄĢå»żĪŖ£ŗ![]() L£¬
L£¬
ŌņøĆČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ£ŗc=n/V=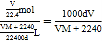 mol”¤L£1£¬
mol”¤L£1£¬
¹ŹŃ”B”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æµē½ā±„ŗĶNaClČÜŅŗŹĒ»Æѧ¹¤ŅµµÄÖŲŅŖ·“Ó¦Ö®Ņ»”£20”ꏱ£¬±„ŗĶNaClČÜŅŗµÄĆܶČĪŖ¦Ńg”¤cm-3(¦Ń£¾1g”¤cm-3)£¬ĪļÖŹµÄĮæÅضČĪŖC mol”¤L-1£¬ČÜÖŹµÄÖŹĮæ·ÖŹżw%£¬ŌņĻĀĮŠĖµ·ØÖŠ²»ÕżČ·µÄŹĒ
A. ĪĀ¶ČøßÓŚ20”ꏱ£¬±„ŗĶNaClČÜŅŗµÄĆܶȓóÓŚ¦Ńg”¤cm-3
B. “ĖČÜŅŗÖŠNaClµÄÖŹĮæ·ÖŹżĪŖ58.5C/(1000¦Ń)%
C. 20”ꏱ£¬ÅØ¶ČŠ”ÓŚC mol”¤L-1µÄNaClČÜŅŗŹĒ²»±„ŗĶČÜŅŗ
D. 20”ꏱ£¬Č”1L±„ŗĶNaClČÜŅŗ£¬¼ÓČėµČĢå»żµÄĖ®Ļ”ŹĶ£¬ĖłµĆČÜŅŗµÄÖŹĮæ·ÖŹż“óÓŚ£Øw/2£©%
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ(1)¢ŁĶ¼I±ķŹ¾10mLĮæĶ²ÖŠŅŗĆęµÄĪ»ÖĆ,AÓėB,BÓėCæĢ¶Č¼äĻą²ī1mL,Čē¹ūæĢ¶ČAŹżÖµĪŖ4,ŌņĮæĶ²ÖŠŅŗĢåµÄĢå»żŹĒ¢Ł,ÓÉÓŚ·ÅÖĆĪ»ÖĆ²»µ±¶ĮŹżŹ±ø©ŹÓ,¶Į³öµÄŹżÖµĪŖ¢ŚŌņ¢Ł¢ŚµÄÕżČ·ŹżÖµĪŖ__________________
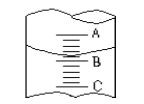
A¢Ł3.2mL”¢¢ŚŠ”ÓŚ3.2mL B.¢Ł4.8mL”¢¢Ś“óÓŚ4.8mL
C.¢Ł3.2mL”¢¢Ś“óÓŚ3.2ml D.¢Ł4.8mL”¢¢ŚŠ”ÓŚ4.8mL
¢ŚŹµŃéŹŅŠčÅäÖĘ1mol”¤L-1µÄNaOHČÜŅŗ220mL,ŌŚÓĆĶŠÅĢĢģĘ½³ĘČ”NaOH¹ĢĢåŹ±,ĢģĘ½¶ĮŹżĪŖ________Ģī“śŗÅ,ĻĀĶ¬)”£
A.“óÓŚ8.8g B.Š”ÓŚ8.8g C.8.8g
£Ø2£©±ķŹ¾ČÜŅŗÅØ¶ČµÄ·½·ØĶس£ÓŠĮ½ÖÖ;ČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż(W)ŗĶĪļÖŹµÄĮæÅØ¶Č (c),Ņņ“ĖŌŚÅäÖĘČÜŅŗŹ±,øł¾Ż²»Ķ¬µÄŠčŅŖ,ÓŠ²»Ķ¬µÄÅäÖĘ·½·Ø”£ĒėĶź³ÉĻĀĮŠĢīæÕĢā”£
¢ń.ÓĆ10%(ĆܶČĪŖ1.01g”¤cm3-)µÄĒāŃõ»ÆÄĘČÜŅŗÅäÖĘ³É27.5g 2%µÄĒāŃõ»ÆÄĘČÜŅŗ”£
¢Ł¼ĘĖć:Šč_________g10%(ĆܶČĪŖ1.01g”¤cm3-)µÄĒāŃõ»ÆÄĘČÜŅŗ
¢ŚĮæČ”:ÓĆĮæĶ²ĮæČ”10%µÄĒāŃõ»ÆÄĘČÜŅŗ_________mL
¢ņ.°Ń98%(ĆܶČĪŖ1.84g”¤cm3-)µÄÅØĮņĖįĻ”ŹĶ³É2mol/LµÄĻ”ĮņĖį100ml,»Ų“šĻĀĮŠĪŹĢā:
¢ŁŠčŅŖĮæČ”ÅØĮņĖį_______ mL
¢ŚĻĀĮŠŹµŃé²Ł×÷Ź¹ÅäÖʵÄČÜŅŗÅضČĘ«µĶµÄŹĒ__________
A.ČŻĮæĘæĻ“µÓŗóĪ“øÉŌļ
B.ĮæČ”ČÜŅŗŹ±,ŃöŹÓæĢ¶ČĻß
C.×°ČėŹŌ¼ĮĘæŹ±,ÓŠÉŁĮæČÜŅŗ½¦³ö
D.ƻӊĻ“µÓÉÕ±ŗĶ²£Į§°ō
E.¶ØČŻŹ±,¼ÓĖ®²»É÷³¬³öæĢ¶ČĻß,ÓÖµ¹³öŅ»Š©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠÓŠ¹ŲĪļÖŹĖ®½āµÄĖµ·ØÕżČ·µÄŹĒ£Ø £©
A.µ°°×ÖŹĖ®½āµÄ×īÖÕ²śĪļŹĒ¶ąėÄ
B.µķ·ŪĖ®½āµÄ×īÖÕ²śĪļŹĒĀóŃæĢĒ
C.ĻĖĪ¬ĖŲ²»ÄÜĖ®½ā³ÉĘĻĢŃĢĒ
D.ÓĶÖ¬Ė®½ā²śĪļÖ®Ņ»ŹĒøŹÓĶ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĻĀĶ¼(ĘäÖŠ¢ń“ś±ķH2A£¬¢ņ“ś±ķHA££¬¢ó“ś±ķA2£)£¬Ļņ20mL0.2mol£ÆL H2AČÜŅŗÖŠµĪ¼Ó0.2mol£ÆL NaOHČÜŅŗ , øł¾ŻĶ¼Ź¾ÅŠ¶Ļ£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)

A. H2AŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ŹĒ£ŗH2A===H£«£«HA££»HA£![]() H£«£«A2£
H£«£«A2£
B. µ±V(NaOH)£½20 mLŹ±£¬ČÜŅŗÖŠø÷Ąė×ÓÅØ¶ČµÄ“óŠ”Ė³ŠņĪŖ£ŗc(Na£«)>c(HA£)>c(H£«)>c(A2£)>c(OH£)
C. µ±V(NaOH)£½30 mLŹ±£¬ČÜŅŗÖŠ“ęŌŚŅŌĻĀ¹ŲĻµ£ŗ2c(H£«)£«c(HA£)£«2c(H2A)£½c(A2£)£«2c(OH£)
D. µČĢå»żµČÅØ¶ČµÄNaOHČÜŅŗÓėH2AČÜŅŗ»ģŗĻŗó£¬ĘäČÜŅŗÖŠĖ®µÄµēĄė³Ģ¶Č±Č“æĖ®“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijŌŖĖŲµÄŌ×ÓÓŠ3øöµē×Ó²ć£¬×īĶā²ćµē×ÓŹżŹĒ6øöµÄŹĒ
A. Ģ¼ B. Ńõ C. Įņ D. µŖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ³£ĪĀĻĀ£¬ĻĀĮŠČÜŅŗÖŠµÄĪ¢Į£ÅØ¶Č¹ŲĻµ²»ÕżČ·µÄŹĒ
A. pH£½8.3µÄijĖįŹ½ŃĪNaHBµÄĖ®ČÜŅŗÖŠ£ŗc (Na+) > c (HB£) > c (H2B) > c (B2£)
B. µČĪļÖŹµÄĮæÅØ¶ČµÄNa2SŗĶNaHSČÜŅŗÖŠ£ŗc (Na+)£½2c (S2£) + c (HS£)
C. NH4HSO4ČÜŅŗÖŠµĪ¼ÓNaOHČÜŅŗÖĮĒ”ŗĆ³ŹÖŠŠŌ£ŗc (Na+) > c (SO42£) > c (NH4+) > c (OH£)£½c (H+)
D. 0.1 mol / L NaH2PO4ČÜŅŗÖŠ£ŗc (Na+)£½c (PO43£) + c (HPO4£²£) + c (H2PO4£) + c (H3PO4)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŌŚAl2 (SO4 )3 ”¢K2 SO4 ŗĶĆ÷·ÆµÄ»ģŗĻČÜŅŗÖŠ,Čē¹ūc(SO4 2- )µČÓŚ0.2 mol/L,µ±¼ÓČėµČĢå»żµÄ0.2 mol/L µÄKOHČÜŅŗŹ±,Éś³ÉµÄ³ĮµķĒ”ŗĆČܽā,ŌņŌ»ģŗĻČÜŅŗÖŠK + µÄĪļÖŹµÄĮæÅضČĪŖ
A. 0.2 mol/L B. 0.25 mol/L C. 0.45 mol/L D. 0.225 mol/L
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ°ŃŅ»Š”æ齚ŹōÄĘ·ÅČėĖ®ÖŠ£¬ĻĀĮŠĻÖĻó²»ÕżČ·µÄŹĒ£Ø £©
A.Naø”ŌŚĖ®ĆęÉĻ
B.NaŌŚĖ®ĆęÉĻÓĪ¶Æ
C.Na³ĮŌŚĖ®ĆęĻĀ
D.NaČŪ³É¹āĮĮµÄŠ”Ēņ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com